Cholinergic and glutamatergic agonists induce gamma frequency activity in dorsal subcoeruleus nucleus neurons
- PMID: 21543743
- PMCID: PMC3154560
- DOI: 10.1152/ajpcell.00093.2011
Cholinergic and glutamatergic agonists induce gamma frequency activity in dorsal subcoeruleus nucleus neurons
Abstract
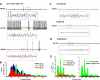
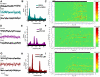
The dorsal subcoeruleus nucleus (SubCD) is involved in generating two signs of rapid eye movement (REM) sleep: muscle atonia and ponto-geniculo-occipital (PGO) waves. We tested the hypothesis that single cell and/or population responses of SubCD neurons are capable of generating gamma frequency activity in response to intracellular stimulation or receptor agonist activation. Whole cell patch clamp recordings (immersion chamber) and population responses (interface chamber) were conducted on 9- to 20-day-old rat brain stem slices. All SubCD neurons (n = 103) fired at gamma frequency when subjected to depolarizing steps. Two statistically distinct populations of neurons were observed, which were distinguished by their high (>80 Hz, n = 24) versus low (35-80 Hz, n = 16) initial firing frequencies. Both cell types exhibited subthreshold oscillations in the gamma range (n = 43), which may underlie the gamma band firing properties of these neurons. The subthreshold oscillations were blocked by the sodium channel blockers tetrodotoxin (TTX, n = 21) extracellularly and N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium bromide (QX-314) intracellularly (n = 5), indicating they were sodium channel dependent. Gamma frequency subthreshold oscillations were observed in response to the nonspecific cholinergic receptor agonist carbachol (CAR, n = 11, d = 1.08) and the glutamate receptor agonists N-methyl-d-aspartic acid (NMDA, n = 12, d = 1.09) and kainic acid (KA, n = 13, d = 0.96), indicating that cholinergic and glutamatergic inputs may be involved in the activation of these subthreshold currents. Gamma band activity also was observed in population responses following application of CAR (n = 4, P < 0.05), NMDA (n = 4, P < 0.05) and KA (n = 4, P < 0.05). Voltage-sensitive, sodium channel-dependent gamma band activity appears to be a part of the intrinsic membrane properties of SubCD neurons.
Figures


References
-
- Azouz R, Gray CM, Nowak LG, McCormick DA. Physiological properties of inhibitory interneurons in cat striate cortex. Cereb Cortex 7:534–545,1997 - PubMed
-
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res 414:245–261,1987 - PubMed
-
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res 306:39–52,1984 - PubMed
-
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci 16:1959–1973,2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

