Krüppel-like factor 9 loss-of-expression in human endometrial carcinoma links altered expression of growth-regulatory genes with aberrant proliferative response to estrogen
- PMID: 21543766
- PMCID: PMC3142261
- DOI: 10.1095/biolreprod.110.090654
Krüppel-like factor 9 loss-of-expression in human endometrial carcinoma links altered expression of growth-regulatory genes with aberrant proliferative response to estrogen
Abstract
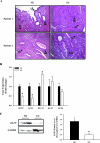
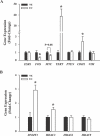
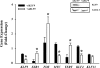
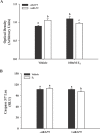
Endometrial cancer is the most commonly diagnosed female genital tract malignancy. Krüppel-like factor 9 (KLF9), a member of the evolutionarily conserved Sp family of transcription factors, is expressed in uterine stroma and glandular epithelium, where it affects cellular proliferation, differentiation, and apoptosis. Deregulated expression of a number of Sp proteins has been associated with multiple types of human tumors, but a role for KLF9 in endometrial cancer development and/or progression is unknown. Here, we evaluated KLF9 expression in endometrial tumors and adjacent uninvolved endometrium of women with endometrial carcinoma. KLF9 mRNA and protein levels were lower in endometrial tumors coincident with decreased expression of family member KLF4 and growth-regulators FBJ murine osteosarcoma viral oncogene homolog (FOS) and myelocytomatosis viral oncogene homolog (MYC) and with increased expression of telomerase reverse transcriptase (TERT) and the chromatin-modifying enzymes DNA methyltransferase 1 (DNMT1) and histone deacetylase 3 (HDAC3). Expression of estrogen receptor alpha (ESR1) and the tumor-suppressor phosphatase and tensin homolog deleted in chromosome 10 (PTEN) did not differ between tumor and normal tissue. The functional relevance of attenuated KLF9 expression in endometrial carcinogenesis was further evaluated in the human endometrial carcinoma cell line Ishikawa by siRNA targeting. KLF9 depletion resulted in loss of normal cellular response to the proliferative effects of estrogen concomitant with reductions in KLF4 and MYC and with enhancement of TERT and ESR1 gene expression. Silencing of KLF4 did not mimic the effects of silencing KLF9 in Ishikawa cells. We suggest that KLF9 loss-of-expression accompanying endometrial carcinogenesis may predispose endometrial epithelial cells to mechanisms of escape from estrogen-mediated growth regulation, leading to progression of established neoplasms.
Figures
References
-
- American Cancer Society. Cancer Facts & Figures 2009. Atlanta: American Cancer Society; 2009.
-
- Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol 2000; 13: 295 308. - PubMed
-
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev 2004; 25: 341 373. - PubMed
-
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 1993; 63: 451 486. - PubMed
-
- Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol 2008; 19: 178 186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

