Mice lacking the USP2 deubiquitinating enzyme have severe male subfertility associated with defects in fertilization and sperm motility
- PMID: 21543767
- PMCID: PMC4480438
- DOI: 10.1095/biolreprod.110.088542
Mice lacking the USP2 deubiquitinating enzyme have severe male subfertility associated with defects in fertilization and sperm motility
Abstract
The ubiquitin-proteasome system plays an important role in spermatogenesis. However, the functions of deubiquitinating enzymes in this process remain poorly characterized. We previously showed that the deubiquitinating enzyme USP2 is induced in late elongating spermatids. To identify its function, we generated mice lacking USP2. Usp2 -/- mice appeared normal, and the weights of major organs, including the testis, did not differ from wild type (Usp2 +/+). However, although the numbers of testicular spermatids and epididymal spermatozoa were normal in Usp2 -/- males, these animals had a severe defect in fertility, yielding only 12% as many offspring as Usp2 +/+ littermates. Spermatogenesis in Usp2 -/- mice was morphologically normal except for the presence of abnormal aggregations of elongating spermatids and formation of multinucleated cells in some tubules. The epididymal epithelium was morphologically normal in Usp2 -/- mice, but some abnormal cells other than sperm were present in the lumen. Usp2 -/- epididymal spermatozoa manifested normal motility when incubated in culture media, but rapidly became immotile when incubated in PBS in contrast to Usp2 +/+ spermatozoa, which largely maintained motility under this condition. Usp2 -/- and +/+ spermatozoa underwent acrosome reactions in vitro with similar frequency. In vitro fertilization assays demonstrated a severe defect in the ability of Usp2 -/- spermatozoa to fertilize eggs. This could be bypassed by intracytoplasmic sperm injection or removal of the zona pellucida, which resulted in fertilization rates similar to that of Usp2 +/+ mice. We demonstrate for the first time, using mouse transgenic approaches, a role for the ubiquitin system in fertilization.
Figures

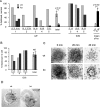



References
-
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 1972; 52: 198 236. - PubMed
-
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells, part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech 2010; 73: 241 278. - PubMed
-
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells, part 2: changes in spermatid organelles associated with development of spermatozoa. Microsc Res Tech 2010; 73: 279 319. - PubMed
-
- Orgebin-Crist MC. Studies on the function of the epididymis. Biol Reprod 1969; 1 (suppl 1): 155 175. - PubMed
-
- Hecht NB. Regulation of 'haploid expressed genes' in male germ cells. J Reprod Fertil 1990; 88: 679 693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

