Structure of Mycobacterium tuberculosis phosphopantetheine adenylyltransferase in complex with the feedback inhibitor CoA reveals only one active-site conformation
- PMID: 21543857
- PMCID: PMC3087636
- DOI: 10.1107/S1744309111010761
Structure of Mycobacterium tuberculosis phosphopantetheine adenylyltransferase in complex with the feedback inhibitor CoA reveals only one active-site conformation
Abstract
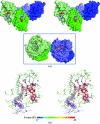
Phosphopantetheine adenylyltransferase (PPAT) catalyzes the penultimate step in the coenzyme A (CoA) biosynthetic pathway, reversibly transferring an adenylyl group from ATP to 4'-phosphopantetheine to form dephosphocoenzyme A (dPCoA). To complement recent biochemical and structural studies on Mycobacterium tuberculosis PPAT (MtPPAT) and to provide further insight into the feedback regulation of MtPPAT by CoA, the X-ray crystal structure of the MtPPAT enzyme in complex with CoA was determined to 2.11 Å resolution. Unlike previous X-ray crystal structures of PPAT-CoA complexes from other bacteria, which showed two distinct CoA conformations bound to the active site, only one conformation of CoA is observed in the MtPPAT-CoA complex.
Figures

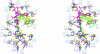
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

