Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum
- PMID: 21544206
- PMCID: PMC3081336
- DOI: 10.1371/journal.pone.0019155
Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum
Abstract
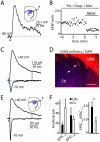
The neurotransmitter glutamate is released by excitatory projection neurons throughout the brain. However, non-glutamatergic cells, including cholinergic and monoaminergic neurons, express markers that suggest that they are also capable of vesicular glutamate release. Striatal cholinergic interneurons (CINs) express the Type-3 vesicular glutamate transporter (VGluT3), although whether they form functional glutamatergic synapses is unclear. To examine this possibility, we utilized mice expressing Cre-recombinase under control of the endogenous choline acetyltransferase locus and conditionally expressed light-activated Channelrhodopsin2 in CINs. Optical stimulation evoked action potentials in CINs and produced postsynaptic responses in medium spiny neurons that were blocked by glutamate receptor antagonists. CIN-mediated glutamatergic responses exhibited a large contribution of NMDA-type glutamate receptors, distinguishing them from corticostriatal inputs. CIN-mediated glutamatergic responses were insensitive to antagonists of acetylcholine receptors and were not seen in mice lacking VGluT3. Our results indicate that CINs are capable of mediating fast glutamatergic transmission, suggesting a new role for these cells in regulating striatal activity.
Conflict of interest statement
Figures


References
-
- Ottersen OP, Storm-Mathisen J. Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol. 1984;229:374–392. - PubMed
-
- Manns ID, Mainville L, Jones BE. Evidence for glutamate, in addition to acetylcholine and GABA, neurotransmitter synthesis in basal forebrain neurons projecting to the entorhinal cortex. Neuroscience. 2001;107:249–263. - PubMed
-
- Kaneko T, Akiyama H, Nagatsu I, Mizuno N. Immunohistochemical demonstration of glutaminase in catecholaminergic and serotoninergic neurons of rat brain. Brain Res. 1990;507:151–154. - PubMed
-
- Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol. 2002;444:191–206. - PubMed
-
- Boulland JL, Qureshi T, Seal RP, Rafiki A, Gundersen V, et al. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480:264–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH50712/MH/NIMH NIH HHS/United States
- P30 DK046200/DK/NIDDK NIH HHS/United States
- DK075632/DK/NIDDK NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- NS063663/NS/NINDS NIH HHS/United States
- R01 DK075632/DK/NIDDK NIH HHS/United States
- R37 MH050712/MH/NIMH NIH HHS/United States
- R01 MH050712/MH/NIMH NIH HHS/United States
- P01 DA010154/DA/NIDA NIH HHS/United States
- DA10154/DA/NIDA NIH HHS/United States
- F31 MH093026/MH/NIMH NIH HHS/United States
- R37 NS046579/NS/NINDS NIH HHS/United States
- NS064984/NS/NINDS NIH HHS/United States
- R01 NS064984/NS/NINDS NIH HHS/United States
- F32 NS063663/NS/NINDS NIH HHS/United States
- NS046579/NS/NINDS NIH HHS/United States
- F32 MH068085/MH/NIMH NIH HHS/United States
- R01 NS046579/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

