Blood pressure effects of naproxcinod in hypertensive patients
- PMID: 21545399
- PMCID: PMC8108868
- DOI: 10.1111/j.1751-7176.2010.00419.x
Blood pressure effects of naproxcinod in hypertensive patients
Abstract
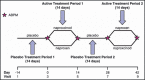
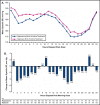
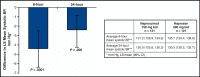
The blood pressure (BP) effects of naproxcinod and naproxen were assessed in an 8-week, double-blind, crossover study in 131 hypertensive patients aged 50 to 74 years. Patients received naproxcinod 750 mg twice daily or naproxen 500 mg twice daily, then the alternate treatment, each for 14 days, with placebo run-in/washout before each active treatment period and 24-hour ambulatory BP monitoring conducted before and after each active treatment period. Mean change from baseline in average 24-hour systolic BP (SBP) after 2 weeks of treatment numerically favored naproxcinod 750 mg twice daily (least-squares [LS] mean for naproxcinod minus naproxen: -1.6 mm Hg; P=.12). Post hoc analyses showed statistically significant SBP differences favoring naproxcinod for the 8 elapsed hours (LS mean: -4.4 mm Hg; P<.0001) and the 24 hours following morning dosing (LS mean: -2.4 mm Hg; P=.006). Naproxcinod may be a beneficial alternative for patients with osteoarthritis requiring nonsteroidal anti-inflammatory drugs.
© 2011 Wiley Periodicals, Inc.
Figures
References
-
- Singh G, Miller JD, Lee FH, et al. Prevalence of cardiovascular disease risk factors among US adults with self‐reported osteoarthritis: data from the Third National Health and Nutrition Examination Survey. Am J Manag Care. 2002;8(15 suppl):S383–S391. - PubMed
-
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. - PubMed
-
- Johnson AG. NSAIDs and blood pressure. Clinical importance for older patients. Drugs Aging. 1998;12:17–27. - PubMed
-
- Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti‐inflammatory drugs affect blood pressure? A meta‐analysis. Ann Intern Med. 1994;121:289–300. - PubMed
-
- Morrison A, Ramey DR, van Adelsberg J, Watson DJ. Systematic review of trials of the effect of continued use of oral non‐selective NSAIDs on blood pressure and hypertension. Curr Med Res Opin. 2007;23:2395–2404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

