Hedgehog signaling controls homeostasis of adult intestinal smooth muscle
- PMID: 21545794
- PMCID: PMC3118277
- DOI: 10.1016/j.ydbio.2011.04.025
Hedgehog signaling controls homeostasis of adult intestinal smooth muscle
Abstract
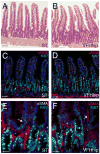
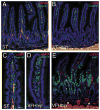
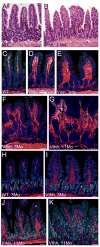
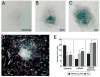
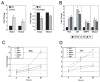
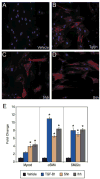
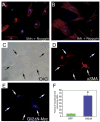
The Hedgehog (Hh) pathway plays multiple patterning roles during development of the mammalian gastrointestinal tract, but its role in adult gut function has not been extensively examined. Here we show that chronic reduction in the combined epithelial Indian (Ihh) and Sonic (Shh) hedgehog signal leads to mislocalization of intestinal subepithelial myofibroblasts, loss of smooth muscle in villus cores and muscularis mucosa as well as crypt hyperplasia. In contrast, chronic over-expression of Ihh in the intestinal epithelium leads to progressive expansion of villus smooth muscle, but does not result in reduced epithelial proliferation. Together, these mouse models show that smooth muscle populations in the adult intestinal lamina propria are highly sensitive to the level of Hh ligand. We demonstrate further that Hh ligand drives smooth muscle differentiation in primary intestinal mesenchyme cultures and that cell-autonomous Hh signal transduction in C3H10T1/2 cells activates the smooth muscle master regulator Myocardin (Myocd) and induces smooth muscle differentiation. The rapid kinetics of Myocd activation by Hh ligands as well as the presence of an unusual concentration of Gli sties in this gene suggest that regulation of Myocd by Hh might be direct. Thus, these data indicate that Hh is a critical regulator of adult intestinal smooth muscle homeostasis and suggest an important link between Hh signaling and Myocd activation. Moreover, the data support the idea that lowered Hh signals promote crypt expansion and increased epithelial cell proliferation, but indicate that chronically increased Hh ligand levels do not dampen crypt proliferation as previously proposed.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–61. - PubMed
-
- Caubit X, Lye CM, Martin E, Core N, Long DA, Vola C, Jenkins D, Garratt AN, Skaer H, Woolf AS, et al. Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development. 2008;135:3301–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

