Prokineticin 1 induces Dickkopf 1 expression and regulates cell proliferation and decidualization in the human endometrium
- PMID: 21546446
- PMCID: PMC3172036
- DOI: 10.1093/molehr/gar031
Prokineticin 1 induces Dickkopf 1 expression and regulates cell proliferation and decidualization in the human endometrium
Abstract
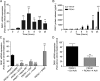
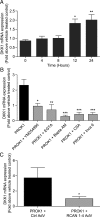
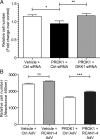
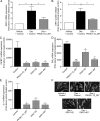
Prokineticin 1 (PROK1) signalling via prokineticin receptor 1 (PROKR1) regulates the expression of several genes with important roles in endometrial receptivity and implantation. This study investigated PROK1 regulation of Dickkopf 1 (DKK1) expression, a negative regulator of canonical Wnt signalling, and its function in the non-pregnant endometrium and first trimester decidua. DKK1 mRNA expression is elevated during the mid-secretory phase of the menstrual cycle and expression increases further in first trimester decidua. DKK1 protein expression is localized to glandular epithelial and stromal cells during the proliferative, early- and mid-secretory phases, whereas expression is confined to the stroma in the late-secretory phase and first trimester decidua. PROK1 induces the expression of DKK1 in endometrial epithelial cells stably expressing PROKR1 and in first trimester decidua explants, via a Gq-calcium-calcineurin-nuclear factor of activated T-cells-mediated pathway. Endometrial epithelial cell proliferation is negatively regulated by PROK1-PROKR1 signalling. We demonstrate that this effect on cell proliferation occurs via DKK1 expression, as siRNA targeted against DKK1 reduces the PROK1-induced decrease in proliferation. Furthermore, decidualization of primary human endometrial stromal cells with progesterone and cyclic adenosine monophosphate is inhibited by miRNA knock down of PROK1 or DKK1. These data demonstrate important roles for PROK1 and DKK1 during endometrial receptivity and early pregnancy, which include regulation of endometrial cell proliferation and decidualization.
Figures

References
-
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. - PubMed
-
- Battersby S, Critchley HO, Morgan K, Millar RP, Jabbour HN. Expression and regulation of the prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2004;89:2463–2469. - PubMed
-
- Borthwick JM, Charnock-Jones DS, Tom BD, Hull ML, Teirney R, Phillips SC, Smith SK. Determination of the transcript profile of human endometrium. Mol Hum Reprod. 2003;9:19–33. - PubMed
-
- Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod. 2002;8:871–879. - PubMed
-
- Chen Q, Zhang Y, Lu J, Wang Q, Wang S, Cao Y, Wang H, Duan E. Embryo-uterine cross-talk during implantation: the role of Wnt signaling. Mol Hum Reprod. 2009;15:215–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

