Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury
- PMID: 21546574
- PMCID: PMC3103724
- DOI: 10.1681/ASN.2010080808
Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury
Abstract
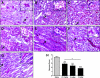
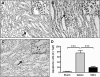
The burst of reactive oxygen species (ROS) during reperfusion of ischemic tissues can trigger the opening of the mitochondrial permeability transition (MPT) pore, resulting in mitochondrial depolarization, decreased ATP synthesis, and increased ROS production. Rapid recovery of ATP upon reperfusion is essential for survival of tubular cells, and inhibition of oxidative damage can limit inflammation. SS-31 is a mitochondria-targeted tetrapeptide that can scavenge mitochondrial ROS and inhibit MPT, suggesting that it may protect against ischemic renal injury. Here, in a rat model of ischemia-reperfusion (IR) injury, treatment with SS-31 protected mitochondrial structure and respiration during early reperfusion, accelerated recovery of ATP, reduced apoptosis and necrosis of tubular cells, and abrogated tubular dysfunction. In addition, SS-31 reduced medullary vascular congestion, decreased IR-mediated oxidative stress and the inflammatory response, and accelerated the proliferation of surviving tubular cells as early as 1 day after reperfusion. In summary, these results support MPT as an upstream target for pharmacologic intervention in IR injury and support early protection of mitochondrial function as a therapeutic maneuver to prevent tubular apoptosis and necrosis, reduce oxidative stress, and reduce inflammation. SS-31 holds promise for the prevention and treatment of acute kidney injury.
Figures
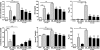

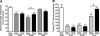




Comment in
-
Pores for thought: new strategies to re-energize stressed mitochondria in acute kidney injury.J Am Soc Nephrol. 2011 Jun;22(6):986-9. doi: 10.1681/ASN.2011030309. Epub 2011 May 12. J Am Soc Nephrol. 2011. PMID: 21566050 No abstract available.
Similar articles
-
Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis.Am J Physiol Renal Physiol. 2014 May 1;306(9):F970-80. doi: 10.1152/ajprenal.00697.2013. Epub 2014 Feb 19. Am J Physiol Renal Physiol. 2014. PMID: 24553434
-
Pores for thought: new strategies to re-energize stressed mitochondria in acute kidney injury.J Am Soc Nephrol. 2011 Jun;22(6):986-9. doi: 10.1681/ASN.2011030309. Epub 2011 May 12. J Am Soc Nephrol. 2011. PMID: 21566050 No abstract available.
-
Protective effects of mitochondrion-targeted peptide SS-31 against hind limb ischemia-reperfusion injury.J Physiol Biochem. 2018 May;74(2):335-343. doi: 10.1007/s13105-018-0617-1. Epub 2018 Mar 27. J Physiol Biochem. 2018. PMID: 29589186
-
Mitochondrial mechanisms and therapeutics in ischaemia reperfusion injury.Pediatr Nephrol. 2019 Jul;34(7):1167-1174. doi: 10.1007/s00467-018-3984-5. Epub 2018 Jun 2. Pediatr Nephrol. 2019. PMID: 29860579 Free PMC article. Review.
-
Mitochondria and ischemia/reperfusion injury.Ann N Y Acad Sci. 2005 Jun;1047:248-58. doi: 10.1196/annals.1341.022. Ann N Y Acad Sci. 2005. PMID: 16093501 Review.
Cited by
-
Youth versus adult-onset type 2 diabetic kidney disease: Insights into currently known structural differences and the potential underlying mechanisms.Clin Sci (Lond). 2022 Nov 11;136(21):1471-1483. doi: 10.1042/CS20210627. Clin Sci (Lond). 2022. PMID: 36326718 Free PMC article.
-
Folate receptor-targeted antioxidant therapy ameliorates renal ischemia-reperfusion injury.J Am Soc Nephrol. 2012 May;23(5):793-800. doi: 10.1681/ASN.2011070711. Epub 2012 Jan 26. J Am Soc Nephrol. 2012. PMID: 22282594 Free PMC article.
-
Disruption of cytochrome c heme coordination is responsible for mitochondrial injury during ischemia.Biochim Biophys Acta. 2015 Oct;1847(10):1075-84. doi: 10.1016/j.bbabio.2015.06.006. Epub 2015 Jun 10. Biochim Biophys Acta. 2015. PMID: 26071084 Free PMC article.
-
Synthetic peptides for the precise transportation of proteins of interests to selectable subcellular areas.Front Bioeng Biotechnol. 2023 Feb 20;11:1062769. doi: 10.3389/fbioe.2023.1062769. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36890909 Free PMC article.
-
Reducing ischemic kidney injury through application of a synchronization modulation electric field to maintain Na+/K+-ATPase functions.Sci Transl Med. 2022 Mar 9;14(635):eabj4906. doi: 10.1126/scitranslmed.abj4906. Epub 2022 Mar 9. Sci Transl Med. 2022. PMID: 35263146 Free PMC article.
References
-
- Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 - PubMed
-
- Wirthensohn G, Guder WG: Renal substrate metabolism. Physiol Rev 66: 469–497, 1986 - PubMed
-
- Klein KL, Wang MS, Torikai S, Davidson WD, Kurokawa K: Substrate oxidation by isolated single nephron segments of the rat. Kidney Int 20: 29–35, 1981 - PubMed
-
- Uchida S, Endou H: Substrate specificity to maintain cellular ATP along the mouse nephron. Am J Physiol 255: F977–F983, 1988 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

