The pro-apoptotic protein Bim is a microRNA target in kidney progenitors
- PMID: 21546576
- PMCID: PMC3103725
- DOI: 10.1681/ASN.2010080841
The pro-apoptotic protein Bim is a microRNA target in kidney progenitors
Abstract
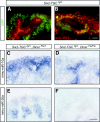
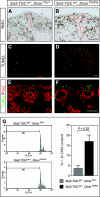
Understanding the mechanisms that regulate nephron progenitors during kidney development should aid development of therapies for renal failure. MicroRNAs, which modulate gene expression through post-transcriptional repression of specific target mRNAs, contribute to the differentiation of stem cells, but their role in nephrogenesis is incompletely understood. Here, we found that the loss of miRNAs in nephron progenitors results in a premature depletion of this population during kidney development. Increased apoptosis and expression of the pro-apoptotic protein Bim accompanied this depletion. Profiling of miRNA expression during nephrogenesis identified several highly expressed miRNAs (miR-10a, miR-106b, miR-17-5p) in nephron progenitors that are either known or predicted to target Bim. We propose that modulation of apoptosis by miRNAs may determine congenital nephron endowment. Furthermore, our data implicate the pro-apoptotic protein Bim as a miRNA target in nephron progenitors.
Figures




References
-
- Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 - PubMed
-
- Schedl A: Renal abnormalities and their developmental origin. Nat Rev Genet 8: 791–802, 2007 - PubMed
-
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ: Dicer is essential for mouse development. Nat Genet 35: 215–217, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

