Dispersal of Group A streptococcal biofilms by the cysteine protease SpeB leads to increased disease severity in a murine model
- PMID: 21547075
- PMCID: PMC3081844
- DOI: 10.1371/journal.pone.0018984
Dispersal of Group A streptococcal biofilms by the cysteine protease SpeB leads to increased disease severity in a murine model
Abstract
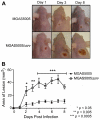
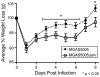
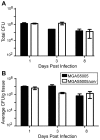
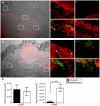
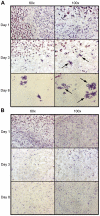
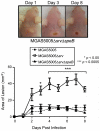
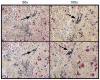
Group A Streptococcus (GAS) is a Gram-positive human pathogen best known for causing pharyngeal and mild skin infections. However, in the 1980's there was an increase in severe GAS infections including cellulitis and deeper tissue infections like necrotizing fasciitis. Particularly striking about this elevation in the incidence of severe disease was that those most often affected were previously healthy individuals. Several groups have shown that changes in gene content or regulation, as with proteases, may contribute to severe disease; yet strains harboring these proteases continue to cause mild disease as well. We and others have shown that group A streptococci (MGAS5005) reside within biofilms both in vitro and in vivo. That is to say that the organism colonizes a host surface and forms a 3-dimensional community encased in a protective matrix of extracellular protein, DNA and polysaccharide(s). However, the mechanism of assembly or dispersal of these structures is unclear, as is the relationship of these structures to disease outcome. Recently we reported that allelic replacement of the streptococcal regulator srv resulted in constitutive production of the streptococcal cysteine protease SpeB. We further showed that the constitutive production of SpeB significantly decreased MGAS5005Δsrv biofilm formation in vitro. Here we show that mice infected with MGAS5005Δsrv had significantly larger lesion development than wild-type infected animals. Histopathology, Gram-staining and immunofluorescence link the increased lesion development with lack of disease containment, lack of biofilm formation, and readily detectable levels of SpeB in the tissue. Treatment of MGAS5005Δsrv infected lesions with a chemical inhibitor of SpeB significantly reduced lesion formation and disease spread to wild-type levels. Furthermore, inactivation of speB in the MGAS5005Δsrv background reduced lesion formation to wild-type levels. Taken together, these data suggest a mechanism by which GAS disease may transition from mild to severe through the Srv mediated dispersal of GAS biofilms.
Conflict of interest statement
Figures


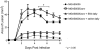
References
-
- Gabillot-Carre M, Roujeau JC. Acute bacterial skin infections and cellulitis. Curr Opin Infect Dis. 2007;20:118–123. - PubMed
-
- Martin JM, Green M. Group A streptococcus. Semin Pediatr Infect Dis. 2006;17:140–148. - PubMed
-
- Rogers RL, Perkins J. Skin and soft tissue infections. Prim Care. 2006;33:697–710. - PubMed
-
- Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–1406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

