Decreased bone formation and osteopenia in lamin a/c-deficient mice
- PMID: 21547077
- PMCID: PMC3081846
- DOI: 10.1371/journal.pone.0019313
Decreased bone formation and osteopenia in lamin a/c-deficient mice
Abstract
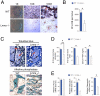
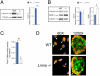
Age-related bone loss is associated with changes in bone cellularity with characteristically low levels of osteoblastogenesis. The mechanisms that explain these changes remain unclear. Although recent in vitro evidence has suggested a new role for proteins of the nuclear envelope in osteoblastogenesis, the role of these proteins in bone cells differentiation and bone metabolism in vivo remains unknown. In this study, we used the lamin A/C null (Lmna⁻/⁻) mice to identify the role of lamin A/C in bone turnover and bone structure in vivo. At three weeks of age, histological and micro computed tomography measurements of femurs in Lmna⁻/⁻ mice revealed a significant decrease in bone mass and microarchitecture in Lmna⁻/⁻ mice as compared with their wild type littermates. Furthermore, quantification of cell numbers after normalization with bone surface revealed a significant reduction in osteoblast and osteocyte numbers in Lmna⁻/⁻ mice compared with their WT littermates. In addition, Lmna⁻/⁻ mice have significantly lower osteoclast number, which show aberrant changes in their shape and size. Finally, mechanistic analysis demonstrated that absence of lamin A/C is associated with increase expression of MAN-1 a protein of the nuclear envelope closely regulated by lamin A/C, which also colocalizes with Runx2 thus affecting its capacity as osteogenic transcription factor. In summary, these data clearly indicate that the presence of lamin A/C is necessary for normal bone turnover in vivo and that absence of lamin A/C induces low bone turnover osteopenia resembling the cellular changes of age-related bone loss.
Conflict of interest statement
Figures


References
-
- Chan GK, Duque G. Age-related bone loss: old bone, new facts. Gerontology. 2002;48:62–71. - PubMed
-
- Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. - PubMed
-
- Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. - PubMed
-
- Kudlow BA, Kennedy BK. Aging: progeria and the lamin connection. Curr Biol. 2010;16:R652–654. - PubMed
-
- Hutchison CJ, Worman HJ. A-type lamins: guardians of the soma? Nat Cell Biol. 2004;6:1062–1067. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

