Dose-dependent effects of dietary fat on development of obesity in relation to intestinal differential gene expression in C57BL/6J mice
- PMID: 21547079
- PMCID: PMC3081848
- DOI: 10.1371/journal.pone.0019145
Dose-dependent effects of dietary fat on development of obesity in relation to intestinal differential gene expression in C57BL/6J mice
Abstract
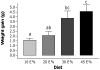
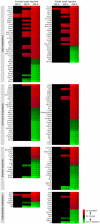
Excessive intake of dietary fat is known to be a contributing factor in the development of obesity. In this study, we determined the dose-dependent effects of dietary fat on the development of this metabolic condition with a focus on changes in gene expression in the small intestine. C57BL/6J mice were fed diets with either 10, 20, 30 or 45 energy% (E%) derived from fat for four weeks (n = 10 mice/diet). We found a significant higher weight gain in mice fed the 30E% and 45E% fat diet compared to mice on the control diet. These data indicate that the main shift towards an obese phenotype lies between a 20E% and 30E% dietary fat intake. Analysis of differential gene expression in the small intestine showed a fat-dose dependent gradient in differentially expressed genes, with the highest numbers in mice fed the 45E% fat diet. The main shift in fat-induced differential gene expression was found between the 30E% and 45E% fat diet. Furthermore, approximately 70% of the differentially expressed genes were changed in a fat-dose dependent manner. Many of these genes were involved in lipid metabolism-related processes and were already differentially expressed on a 30E% fat diet. Taken together, we conclude that up to 20E% of dietary fat, the small intestine has an effective 'buffer capacity' for fat handling. From 30E% of dietary fat, a switch towards an obese phenotype is triggered. We further speculate that especially fat-dose dependently changed lipid metabolism-related genes are involved in development of obesity.
Conflict of interest statement
Figures



Similar articles
-
Nonlinear transcriptomic response to dietary fat intake in the small intestine of C57BL/6J mice.BMC Genomics. 2016 Feb 9;17:106. doi: 10.1186/s12864-016-2424-9. BMC Genomics. 2016. PMID: 26861690 Free PMC article.
-
Supplementary dietary calcium stimulates faecal fat and bile acid excretion, but does not protect against obesity and insulin resistance in C57BL/6J mice.Br J Nutr. 2011 Apr;105(7):1005-11. doi: 10.1017/S0007114510004654. Epub 2010 Dec 23. Br J Nutr. 2011. PMID: 21205428
-
Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine.Am J Physiol Gastrointest Liver Physiol. 2012 Sep 1;303(5):G589-99. doi: 10.1152/ajpgi.00488.2011. Epub 2012 Jun 14. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22700822
-
Pharmacological Inhibition of Monoacylglycerol O-Acyltransferase 2 Improves Hyperlipidemia, Obesity, and Diabetes by Change in Intestinal Fat Utilization.PLoS One. 2016 Mar 3;11(3):e0150976. doi: 10.1371/journal.pone.0150976. eCollection 2016. PLoS One. 2016. PMID: 26938273 Free PMC article.
-
The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice.BMC Med Genomics. 2008 May 6;1:14. doi: 10.1186/1755-8794-1-14. BMC Med Genomics. 2008. PMID: 18457598 Free PMC article.
Cited by
-
Therapeutic effects of intermittent fasting on high-fat, high-fructose diet; involvement of jejunal aquaporin 1, 3, and 7.Heliyon. 2024 Mar 20;10(7):e28436. doi: 10.1016/j.heliyon.2024.e28436. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38560252 Free PMC article.
-
Intestinal miRNAs regulated in response to dietary lipids.Sci Rep. 2020 Nov 3;10(1):18921. doi: 10.1038/s41598-020-75751-w. Sci Rep. 2020. PMID: 33144601 Free PMC article.
-
Obesity, independent of diet, drives lasting effects on intestinal epithelial stem cell proliferation in mice.Exp Biol Med (Maywood). 2018 Jun;243(10):826-835. doi: 10.1177/1535370218777762. Exp Biol Med (Maywood). 2018. PMID: 29932373 Free PMC article.
-
Deletion of intestinal epithelial insulin receptor attenuates high-fat diet-induced elevations in cholesterol and stem, enteroendocrine, and Paneth cell mRNAs.Am J Physiol Gastrointest Liver Physiol. 2015 Jan 15;308(2):G100-11. doi: 10.1152/ajpgi.00287.2014. Epub 2014 Nov 13. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25394660 Free PMC article.
-
Increased plasma citrulline in mice marks diet-induced obesity and may predict the development of the metabolic syndrome.PLoS One. 2013 May 14;8(5):e63950. doi: 10.1371/journal.pone.0063950. Print 2013. PLoS One. 2013. PMID: 23691124 Free PMC article.
References
-
- Parekh PI, Petro AE, Tiller JM, Feinglos MN, Surwit RS. Reversal of diet-induced obesity and diabetes in C57BL/6J mice. Metabolism. 1998;47:1089–1096. - PubMed
-
- Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–219. - PubMed
-
- Roche HM, Phillips C, Gibney MJ. The metabolic syndrome: the crossroads of diet and genetics. Proc Nutr Soc. 2005;64:371–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

