Integrin targeted therapeutics
- PMID: 21547158
- PMCID: PMC3086618
- DOI: 10.7150/thno/v01p0154
Integrin targeted therapeutics
Abstract
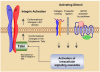
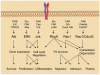
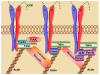
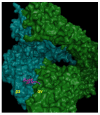
Integrins are heterodimeric, transmembrane receptors that function as mechanosensors, adhesion molecules and signal transduction platforms in a multitude of biological processes. As such, integrins are central to the etiology and pathology of many disease states. Therefore, pharmacological inhibition of integrins is of great interest for the treatment and prevention of disease. In the last two decades several integrin-targeted drugs have made their way into clinical use, many others are in clinical trials and still more are showing promise as they advance through preclinical development. Herein, this review examines and evaluates the various drugs and compounds targeting integrins and the disease states in which they are implicated.
Keywords: Crohn's Disease; Integrin-targeted therapeutics; Multiple Sclerosis; abciximab; abegrin; acute coronary syndromes; age related macular degeneration; angiogenesis; cancer; cilengitide; eptifibatide; integrin-targeted peptides; integrin-targeted small molecules; integrin-targeted therapeutic antibodies; natalizumab; osteoporosis; tirofiban.
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
Figures


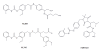
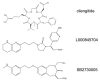
References
-
- Shimaoka M, Springer T. Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov. 2003;2:703–16. - PubMed
-
- Hynes R. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. - PubMed
-
- Legate K, Wickström S, Fässler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

