Predicting treatment outcomes and responder subsets in scleroderma-related interstitial lung disease
- PMID: 21547897
- PMCID: PMC3910296
- DOI: 10.1002/art.30438
Predicting treatment outcomes and responder subsets in scleroderma-related interstitial lung disease
Abstract
Objective: To identify baseline characteristics of patients with scleroderma-related interstitial lung disease (SSc-ILD) that could serve as predictors of the most favorable response to 12-month treatment with oral cyclophosphamide (CYC).
Methods: Regression analyses were retrospectively applied to the Scleroderma Lung Study data in order to identify baseline characteristics that correlated with the absolute change in forced vital capacity (FVC) (% predicted values) and the placebo-adjusted change in % predicted FVC over time (the CYC treatment effect).
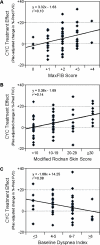
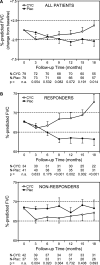
Results: Completion of the CYC arm of the Scleroderma Lung Study was associated with a placebo-adjusted improvement in the % predicted FVC of 2.11% at 12 months, which increased to 4.16% when patients were followed up for another 6 months (P=0.014). Multivariate regression analyses identified the maximal severity of reticular infiltrates (assessed as maximum fibrosis scores) on high-resolution computed tomography (HRCT) at baseline, the modified Rodnan skin thickness score (MRSS) at baseline, and the Mahler baseline dyspnea index as independent correlates of treatment response. When patients were stratified on the basis of whether 50% or more of any lung zone was involved by reticular infiltrates on HRCT and/or whether patients exhibited an MRSS of at least 23, a subgroup of patients emerged in whom there was an average CYC treatment effect of 9.81% at 18 months (P<0.001). Conversely, there was no treatment effect (a -0.58% difference) in patients with less severe HRCT findings and a lower MRSS at baseline.
Conclusion: A retrospective analysis of the Scleroderma Lung Study data identified the severity of reticular infiltrates on baseline HRCT and the baseline MRSS as patient features that might be predictive of responsiveness to CYC therapy.
Trial registration: ClinicalTrials.gov NCT00004563.
Copyright © 2011 by the American College of Rheumatology.
Figures
References
-
- Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–70. - PubMed
-
- Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–66. - PubMed
-
- Martinez FJ, McCune WJ. Cyclophosphamide for scleroderma lung disease. N Engl J Med. 2006;354(25):2707–09. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 HL060794/HL/NHLBI NIH HHS/United States
- R01 HL089901/HL/NHLBI NIH HHS/United States
- U01-HL-60597/HL/NHLBI NIH HHS/United States
- U01-HL-60748/HL/NHLBI NIH HHS/United States
- U01-HL-60750/HL/NHLBI NIH HHS/United States
- U01-HL-60823/HL/NHLBI NIH HHS/United States
- U01-HL-60550/HL/NHLBI NIH HHS/United States
- U01 HL060606/HL/NHLBI NIH HHS/United States
- U01 HL060597/HL/NHLBI NIH HHS/United States
- U01-HL-60587/HL/NHLBI NIH HHS/United States
- U01 HL060682/HL/NHLBI NIH HHS/United States
- U01 HL060839/HL/NHLBI NIH HHS/United States
- R01-HL-089901/HL/NHLBI NIH HHS/United States
- R01 HL089758/HL/NHLBI NIH HHS/United States
- U01-HL-60794/HL/NHLBI NIH HHS/United States
- U01 HL060587/HL/NHLBI NIH HHS/United States
- U01-HL-60682/HL/NHLBI NIH HHS/United States
- R01-AR-055075/AR/NIAMS NIH HHS/United States
- U01-HL-60895/HL/NHLBI NIH HHS/United States
- R01-HL-089758/HL/NHLBI NIH HHS/United States
- R01 AR055075/AR/NIAMS NIH HHS/United States
- U01 HL060550/HL/NHLBI NIH HHS/United States
- U01-HL-60839/HL/NHLBI NIH HHS/United States
- U01 HL060895/HL/NHLBI NIH HHS/United States
- U01 HL060750/HL/NHLBI NIH HHS/United States
- U01-HL-60606/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

