Mild redox complementation enables H2 activation by [FeFe]-hydrogenase models
- PMID: 21548619
- PMCID: PMC3115929
- DOI: 10.1021/ja201731q
Mild redox complementation enables H2 activation by [FeFe]-hydrogenase models
Abstract
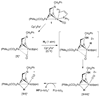
Mild oxidants such as [Fe(C(5)Me(5))(2)](+) accelerate the activation of H(2) by [Fe(2)[(SCH(2))(2)NBn](CO)(3)(dppv)(PMe(3))](+) ([1](+)), despite the fact that the ferrocenium cation is incapable of oxidizing [1](+). The reaction is first-order in [1](+) and [H(2)] but independent of the E(1/2) and concentration of the oxidant. The analogous reaction occurs with D(2) and proceeds with an inverse kinetic isotope effect of 0.75(8). The activation of H(2) is further enhanced with the tetracarbonyl [Fe(2)[(SCH(2))(2)NBn](CO)(4)(dppn)](+) ([2](+)), the first crystallographically characterized model for the H(ox) state of the active site containing an amine cofactor. These studies point to rate-determining binding of H(2) followed by proton-coupled electron transfer. Relative to that by [1](+), the rate of H(2) activation by [2](+)/Fc(+) is enhanced by a factor of 10(4) at 25 °C.
© 2011 American Chemical Society
Figures





References
-
- Rakowski Dubois M, Dubois DL. Acct. Chem. Res. 2009;42:1974–1982. - PubMed
- Karunadasa HI, Chang CJ, Long JR. Nature. 2010;464:1329–1333. - PubMed
- Yang JY, Chen S, Dougherty WG, Kassel WS, Bullock RM, DuBois DL, Raugei S, Rousseau R, Dupuis M, Rakowski DuBois M. Chem. Commun. 2010;46:8618–8620. - PubMed
- Dempsey JL, Brunschwig BS, Winkler JR, Gray HB. Acc. Chem. Res. 2009;42:1995–2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

