High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus
- PMID: 21548924
- PMCID: PMC3218880
- DOI: 10.1186/ar3332
High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus
Abstract
Introduction: High Mobility Group Box 1 (HMGB1) is a nuclear non-histone protein. HMGB1, which is secreted by inflammatory cells and passively released from apoptotic and necrotic cells, may act as a pro-inflammatory mediator. As apoptotic cells accumulate in systemic lupus erythematosus (SLE), HMGB1 levels might be increased in SLE. HMGB1 may also serve as an autoantigen, leading to the production of anti-HMGB1 antibodies. In this study we determined levels of HMGB1 and anti-HMGB1 in SLE patients in comparison to healthy controls (HC) and analysed their relation with disease activity.
Methods: The study population consisted of 70 SLE patients and 35 age- and sex-matched HC. Thirty-three SLE patients had quiescent disease, the other 37 patients were selected for having active disease. Nineteen of these had lupus nephritis. HMGB1 levels were measured with both Western blot and ELISA. Anti-HMGB1 levels were measured by ELISA. Clinical and serological parameters were assessed according to routine procedures.
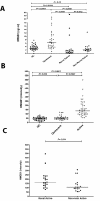
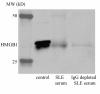
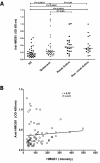
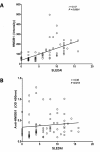
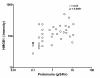
Results: HMGB1 levels in SLE patients could be measured reliably by Western blotting only, and were significantly increased compared to HC. During active disease HMGB1 levels increased, in particular in patients with renal involvement. Serum HMGB1 levels correlated with SLEDAI, proteinuria, and anti-dsDNA levels, and showed a negative correlation with complement C3. Anti-HMGB1 levels were significantly increased in SLE patients compared to HC, and positively correlated with HMGB1 levels.
Conclusions: Levels of HMGB1 in the sera of SLE patients, in particular in those with active renal disease, are increased. Serum HMGB1 levels are related to SLEDAI scores and proteinuria, as well as to levels of anti-HMGB1 antibodies. These findings suggest that besides HMGB1, HMGB1-anti-HMGB1 immune complexes play a role in the pathogenesis of SLE, in particular in patients with renal involvement.
Figures

References
-
- Bergtold A, Gavhane A, D'Agati V, Madaio M, Clynes R. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J Immunol. 2006;177:7287–7295. - PubMed
-
- Tokumoto M, Fukuda K, Shinozaki M, Kashiwagi M, Katafuchi R, Yoshida T, Yanagida T, Kanai H, Hirakata H, Tamaki K, Okuda S, Fujishima M. Acute interstitial nephritis with immune complex deposition and MHC class II antigen presentation along the tubular basement membrane. Nephrol Dial Transplant. 1999;14:2210–2215. doi: 10.1093/ndt/14.9.2210. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

