Pathogenesis of Lassa fever in cynomolgus macaques
- PMID: 21548931
- PMCID: PMC3104370
- DOI: 10.1186/1743-422X-8-205
Pathogenesis of Lassa fever in cynomolgus macaques
Abstract
Background: Lassa virus (LASV) infection causes an acute and sometimes fatal hemorrhagic disease in humans and nonhuman primates; however, little is known about the development of Lassa fever. Here, we performed a pilot study to begin to understand the progression of LASV infection in nonhuman primates.
Methods: Six cynomolgus monkeys were experimentally infected with LASV. Tissues from three animals were examined at an early- to mid-stage of disease and compared with tissues from three animals collected at terminal stages of disease.
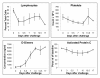
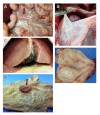
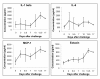
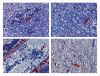
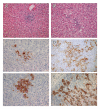
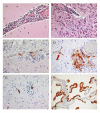
Results: Dendritic cells were identified as a prominent target of LASV infection in a variety of tissues in all animals at day 7 while Kupffer cells, hepatocytes, adrenal cortical cells, and endothelial cells were more frequently infected with LASV in tissues of terminal animals (days 13.5-17). Meningoencephalitis and neuronal necrosis were noteworthy findings in terminal animals. Evidence of coagulopathy was noted; however, the degree of fibrin deposition in tissues was less prominent than has been reported in other viral hemorrhagic fevers.
Conclusion: The sequence of pathogenic events identified in this study begins to shed light on the development of disease processes during Lassa fever and also may provide new targets for rational prophylactic and chemotherapeutic interventions.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

