Biochemical properties of pancreatic colipase from the common stingray Dasyatis pastinaca
- PMID: 21549005
- PMCID: PMC3098174
- DOI: 10.1186/1476-511X-10-69
Biochemical properties of pancreatic colipase from the common stingray Dasyatis pastinaca
Abstract
Background: Pancreatic colipase is a required co-factor for pancreatic lipase, being necessary for its activity during hydrolysis of dietary triglycerides in the presence of bile salts. In the intestine, colipase is cleaved from a precursor molecule, procolipase, through the action of trypsin. This cleavage yields a peptide called enterostatin knoswn, being produced in equimolar proportions to colipase.
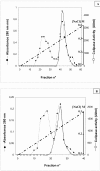
Results: In this study, colipase from the common stingray Dasyatis pastinaca (CoSPL) was purified to homogeneity. The purified colipase is not glycosylated and has an apparent molecular mass of around 10 kDa. The NH2-terminal sequencing of purified CoSPL exhibits more than 55% identity with those of mammalian, bird or marine colipases. CoSPL was found to be less effective activator of bird and mammal pancreatic lipases than for the lipase from the same specie. The apparent dissociation constant (Kd) of the colipase/lipase complex and the apparent Vmax of the colipase-activated lipase values were deduced from the linear curves of the Scatchard plots. We concluded that Stingray Pancreatic Lipase (SPL) has higher ability to interact with colipase from the same species than with the mammal or bird ones.
Conclusion: The fact that colipase is a universal lipase cofactor might thus be explained by a conservation of the colipase-lipase interaction site. The results obtained in the study may improve our knowledge of marine lipase/colipase.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

