Design and characterization of epitope-scaffold immunogens that present the motavizumab epitope from respiratory syncytial virus
- PMID: 21549714
- PMCID: PMC3107930
- DOI: 10.1016/j.jmb.2011.04.044
Design and characterization of epitope-scaffold immunogens that present the motavizumab epitope from respiratory syncytial virus
Abstract
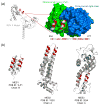
Respiratory syncytial virus (RSV) is a major cause of respiratory tract infections in infants, but an effective vaccine has not yet been developed. An ideal vaccine would elicit protective antibodies while avoiding virus-specific T-cell responses, which have been implicated in vaccine-enhanced disease with previous RSV vaccines. We propose that heterologous proteins designed to present RSV-neutralizing antibody epitopes and to elicit cognate antibodies have the potential to fulfill these vaccine requirements, as they can be fashioned to be free of viral T-cell epitopes. Here we present the design and characterization of three epitope-scaffolds that present the epitope of motavizumab, a potent neutralizing antibody that binds to a helix-loop-helix motif in the RSV fusion glycoprotein. Two of the epitope-scaffolds could be purified, and one epitope-scaffold based on a Staphylococcus aureus protein A domain bound motavizumab with kinetic and thermodynamic properties consistent with the free epitope-scaffold being stabilized in a conformation that closely resembled the motavizumab-bound state. This epitope-scaffold was well folded as assessed by circular dichroism and isothermal titration calorimetry, and its crystal structure (determined in complex with motavizumab to 1.9 Å resolution) was similar to the computationally designed model, with all hydrogen-bond interactions critical for binding to motavizumab preserved. Immunization of mice with this epitope-scaffold failed to elicit neutralizing antibodies but did elicit sera with F binding activity. The elicitation of F binding antibodies suggests that some of the design criteria for eliciting protective antibodies without virus-specific T-cell responses are being met, but additional optimization of these novel immunogens is required.
Published by Elsevier Ltd.
Figures




References
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EAF, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. The Lancet. 2010;375:1545–1555. - PMC - PubMed
-
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory Syncytial Virus Disease in Infants Despite Prior Administration of Antigenic Inactivated Vaccine. Am J Epidemiol. 1969;89:422–434. - PubMed
-
- Graham B, Henderson G, Tang Y, Lu X, Neuzil K, Colley D. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

