Elastin-like protein matrix reinforced with collagen microfibers for soft tissue repair
- PMID: 21550111
- PMCID: PMC3207740
- DOI: 10.1016/j.biomaterials.2011.04.009
Elastin-like protein matrix reinforced with collagen microfibers for soft tissue repair
Abstract
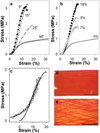
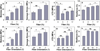
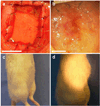
Artificial composites designed to mimic the structure and properties of native extracellular matrix may lead to acellular materials for soft tissue repair and replacement, which display mechanical strength, stiffness, and resilience resembling native tissue. We describe the fabrication of thin lamellae consisting of continuous collagen microfiber embedded at controlled orientations and densities in a recombinant elastin-like protein polymer matrix. Multilamellar stacking affords flexible, protein-based composite sheets whose properties are dependent upon both the elastomeric matrix and collagen content and organization. Sheets are produced with properties that range over 13-fold in elongation to break (23-314%), six-fold in Young's modulus (5.3-33.1 MPa), and more than two-fold in tensile strength (1.85-4.08 MPa), exceeding that of a number of native human tissues, including urinary bladder, pulmonary artery, and aorta. A sheet approximating the mechanical response of human abdominal wall fascia is investigated as a fascial substitute for ventral hernia repair. Protein-based composite patches prevent hernia recurrence in Wistar rats over an 8-week period with new tissue formation and sustained structural integrity.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Generation of spatially aligned collagen fiber networks through microtransfer molding.Adv Healthc Mater. 2014 Mar;3(3):367-74. doi: 10.1002/adhm.201300112. Epub 2013 Aug 29. Adv Healthc Mater. 2014. PMID: 24039146 Free PMC article.
-
The use of microfiber composites of elastin-like protein matrix reinforced with synthetic collagen in the design of vascular grafts.Biomaterials. 2010 Sep;31(27):7175-82. doi: 10.1016/j.biomaterials.2010.05.014. Epub 2010 Jun 26. Biomaterials. 2010. PMID: 20584549 Free PMC article.
-
Biomimetic collagen/elastin meshes for ventral hernia repair in a rat model.Acta Biomater. 2017 Mar 1;50:165-177. doi: 10.1016/j.actbio.2016.11.032. Epub 2016 Nov 18. Acta Biomater. 2017. PMID: 27872012
-
Fabricated Elastin.Adv Healthc Mater. 2015 Nov 18;4(16):2530-2556. doi: 10.1002/adhm.201400781. Epub 2015 Mar 13. Adv Healthc Mater. 2015. PMID: 25771993 Free PMC article. Review.
-
Elastic materials for tissue engineering applications: Natural, synthetic, and hybrid polymers.Acta Biomater. 2018 Oct 1;79:60-82. doi: 10.1016/j.actbio.2018.08.027. Epub 2018 Aug 28. Acta Biomater. 2018. PMID: 30165203 Review.
Cited by
-
A depth dependent transversely isotropic micromechanic model of articular cartilage.J Mater Sci Mater Med. 2015 Feb;26(2):111. doi: 10.1007/s10856-015-5449-8. Epub 2015 Feb 11. J Mater Sci Mater Med. 2015. PMID: 25665849
-
Continuous Formation of Ultrathin, Strong Collagen Sheets with Tunable Anisotropy and Compaction.ACS Biomater Sci Eng. 2020 Jul 13;6(7):4236-4246. doi: 10.1021/acsbiomaterials.0c00321. Epub 2020 May 26. ACS Biomater Sci Eng. 2020. PMID: 32685675 Free PMC article.
-
The application of fiber-reinforced materials in disc repair.Biomed Res Int. 2013;2013:714103. doi: 10.1155/2013/714103. Epub 2013 Dec 8. Biomed Res Int. 2013. PMID: 24383057 Free PMC article. Review.
-
Microfabricated intracortical extracellular matrix-microelectrodes for improving neural interfaces.Microsyst Nanoeng. 2018 Sep 24;4:30. doi: 10.1038/s41378-018-0030-5. eCollection 2018. Microsyst Nanoeng. 2018. PMID: 31057918 Free PMC article.
-
General theory of skin reinforcement.PLoS One. 2017 Aug 10;12(8):e0182865. doi: 10.1371/journal.pone.0182865. eCollection 2017. PLoS One. 2017. PMID: 28797059 Free PMC article.
References
-
- Shadwick RE. Mechanical design in arteries. J Exp Biol. 1999;202:3305–3313. - PubMed
-
- Roach MR, Burton AC. The reason for the shape of the distensibility curves of arteries. Can J Biochem Physiol. 1957;35:681–690. - PubMed
-
- Vesely I. The role of elastin in aortic valve mechanics. J Biomech. 1998;31:115–123. - PubMed
-
- Silver FH, Freeman JW, DeVore D. Viscoelastic properties of human skin and processed dermis. Skin Res Technol. 2001;7:18–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

