Neonatal tissue damage facilitates nociceptive synaptic input to the developing superficial dorsal horn via NGF-dependent mechanisms
- PMID: 21550171
- PMCID: PMC3139000
- DOI: 10.1016/j.pain.2011.04.001
Neonatal tissue damage facilitates nociceptive synaptic input to the developing superficial dorsal horn via NGF-dependent mechanisms
Abstract
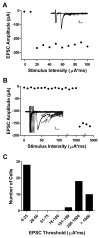
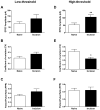
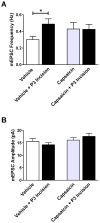
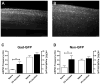
Tissue injury during a critical period of early life can facilitate spontaneous glutamatergic transmission within developing pain circuits in the superficial dorsal horn (SDH) of the spinal cord. However, the extent to which neonatal tissue damage strengthens nociceptive synaptic input to specific subpopulations of SDH neurons, as well as the mechanisms underlying this distinct form of synaptic plasticity, remains unclear. Here we use in vitro whole-cell patch clamp recordings from rodent spinal cord slices to demonstrate that neonatal surgical injury selectively potentiates high-threshold primary afferent input to immature lamina II neurons. In addition, the increase in the frequency of miniature excitatory postsynaptic currents after hindpaw incision was prevented by neonatal capsaicin treatment, suggesting that early tissue injury enhances glutamate release from nociceptive synapses. This occurs in a widespread manner within the developing SDH, as incision elevated miniature excitatory postsynaptic current frequency in both GABAergic and presumed glutamatergic lamina II neurons of Gad-GFP transgenic mice. The administration of exogenous nerve growth factor into the rat hindpaw mimicked the effects of early tissue damage on excitatory synaptic function, while blocking trkA receptors in vivo abolished the changes in both spontaneous and primary afferent-evoked glutamatergic transmission following incision. These findings illustrate that neonatal tissue damage can alter the gain of developing pain pathways by activating nerve growth factor-dependent signaling cascades, which modify synaptic efficacy at the first site of nociceptive processing within the central nervous system.
Copyright © 2011 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest with any of the work presented in this manuscript.
Figures



References
-
- Alexander J, Manno M. Underuse of analgesia in very young pediatric patients with isolated painful injuries. Ann Emerg Med. 2003;41:617–622. - PubMed
-
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. - PubMed
-
- Anand KJ. Pain, plasticity, and premature birth: a prescription for permanent suffering? Nat Med. 2000;6:971–973. - PubMed
-
- Andrews K, Fitzgerald M. The cutaneous withdrawal reflex in human neonates: sensitization, receptive fields, and the effects of contralateral stimulation. Pain. 1994;56:95–101. - PubMed
-
- Andrews KA, Desai D, Dhillon HK, Wilcox DT, Fitzgerald M. Abdominal sensitivity in the first year of life: comparison of infants with and without prenatally diagnosed unilateral hydronephrosis. Pain. 2002;100:35–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

