Functional and metabolic effects of adaptive glycerol kinase (GLPK) mutants in Escherichia coli
- PMID: 21550976
- PMCID: PMC3123082
- DOI: 10.1074/jbc.M110.195305
Functional and metabolic effects of adaptive glycerol kinase (GLPK) mutants in Escherichia coli
Abstract
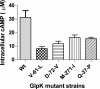
Herein we measure the effect of four adaptive non-synonymous mutations to the glycerol kinase (glpK) gene on catalytic function and regulation, to identify changes that correlate to increased fitness in glycerol media. The mutations significantly reduce affinity for the allosteric inhibitor fructose-1,6-bisphosphate (FBP) and formation of the tetramer, which are structurally related, in a manner that correlates inversely with imparted fitness during growth on glycerol, which strongly suggests that these enzymatic parameters drive growth improvement. Counterintuitively, the glpK mutations also increase glycerol-induced auto-catabolite repression that reduces glpK transcription in a manner that correlates to fitness. This suggests that increased specific GlpK activity is attenuated by negative feedback on glpK expression via catabolite repression, possibly to prevent methylglyoxal toxicity. We additionally report that glpK mutations were fixed in 47 of 50 independent glycerol-adapted lineages. By far the most frequently mutated locus (nucleotide 218) was mutated in 20 lineages, strongly suggesting this position has an elevated mutation rate. This study demonstrates that fitness correlations can be used to interrogate adaptive processes at the protein level and to identify the regulatory constraints underlying selection and improved growth.
Figures


References
-
- Knight C. G., Zitzmann N., Prabhakar S., Antrobus R., Dwek R., Hebestreit H., Rainey P. B. (2006) Nat. Genet. 38, 1015–1022 - PubMed
-
- Lin E. C. (1976) Annu. Rev. Microbiol. 30, 535–578 - PubMed
-
- Herring C. D., Raghunathan A., Honisch C., Patel T., Applebee M. K., Joyce A. R., Albert T. J., Blattner F. R., van den Boom D., Cantor C. R., Palsson B. Ø. (2006) Nat. Genet. 38, 1406–1412 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

