Clinical efficacy and cost-effectiveness of lanthanum carbonate as second-line therapy in hemodialysis patients in Japan
- PMID: 21551021
- PMCID: PMC3109935
- DOI: 10.2215/CJN.08841010
Clinical efficacy and cost-effectiveness of lanthanum carbonate as second-line therapy in hemodialysis patients in Japan
Abstract
Background and objectives: Lanthanum carbonate (LC) is a nonaluminum, noncalcium phosphate binder that is effective for hyperphosphatemia in dialysis patients. However, its efficacy and cost-effectiveness as second-line therapy have not been fully examined.
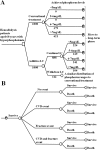
Design, setting, participants, & measurements: We first conducted a multicenter, open-label, 16-week clinical trial to examine the effect of additive LC in 116 hemodialysis patients who had uncontrolled hyperphosphatemia with conventional phosphorus-lowering therapy alone. Based on these clinical data, a state transition model was developed to evaluate the benefits and costs associated with LC as second-line therapy. Reduced risks for cardiovascular morbidity and mortality among patients treated with LC arise through more of the population achieving the target phosphorus levels. Uncertainty was explored through sensitivity analysis.
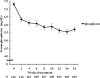
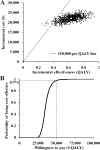
Results: After 16 weeks of additive LC treatment, mean serum phosphorus levels decreased from 7.30 ± 0.90 to 5.71 ± 1.32 mg/dl, without significant changes in serum calcium or intact parathyroid hormone levels. A subsequent cost-effectiveness analysis showed that compared with conventional treatment, additive LC incurred an average additional lifetime cost of $22,054 per person and conferred an additional 0.632 quality-adjusted life years (QALYs). This resulted in an incremental cost-effectiveness ratio of $34,896 per QALY gained. Applying a cost-effectiveness threshold of $50,000 per QALY, a probabilistic sensitivity analysis showed that additive LC had a 97.4% probability of being cost-effective compared with conventional treatment.
Conclusions: Our results indicate that the use of LC as second-line therapy would be cost-effective among hemodialysis patients with uncontrolled hyperphosphatemia in Japan.
Figures
Similar articles
-
Cost-effectiveness of lanthanum carbonate in the treatment of hyperphosphatemia in dialysis patients: a Canadian payer perspective.Clin Ther. 2012 Jul;34(7):1531-43. doi: 10.1016/j.clinthera.2012.06.006. Epub 2012 Jun 27. Clin Ther. 2012. PMID: 22742885
-
Cost-effectiveness of lanthanum carbonate versus sevelamer hydrochloride for the treatment of hyperphosphatemia in patients with end-stage renal disease: a US payer perspective.Value Health. 2011 Dec;14(8):1002-9. doi: 10.1016/j.jval.2011.05.043. Epub 2011 Jul 28. Value Health. 2011. PMID: 22152168
-
Cost-effectiveness of lanthanum carbonate in the treatment of hyperphosphatemia in chronic kidney disease before and during dialysis.Value Health. 2011 Sep-Oct;14(6):852-8. doi: 10.1016/j.jval.2011.05.005. Epub 2011 Jun 23. Value Health. 2011. PMID: 21914505
-
A systematic review of the economic evaluations of non-calcium-containing phosphate binders, sevelamer and Lanthanum, in end-stage renal disease patients with hyperphosphatemia.Expert Rev Pharmacoecon Outcomes Res. 2019 Jun;19(3):287-298. doi: 10.1080/14737167.2019.1567336. Epub 2019 Jan 21. Expert Rev Pharmacoecon Outcomes Res. 2019. PMID: 30664365
-
Effect of Lanthanum Carbonate on All-Cause Mortality in Patients Receiving Maintenance Hemodialysis: a Meta-Analysis of Randomized Controlled Trials.Kidney Blood Press Res. 2018;43(2):536-544. doi: 10.1159/000488700. Epub 2018 Mar 29. Kidney Blood Press Res. 2018. PMID: 29627829 Review.
Cited by
-
Medical expenditures for fragility hip fracture in Japan: a study using the nationwide health insurance claims database.Arch Osteoporos. 2022 Apr 11;17(1):61. doi: 10.1007/s11657-022-01096-8. Arch Osteoporos. 2022. PMID: 35403938 Free PMC article.
-
The management of hyperphosphatemia by lanthanum carbonate in chronic kidney disease patients.Int J Nephrol Renovasc Dis. 2012;5:81-9. doi: 10.2147/IJNRD.S15466. Epub 2012 May 29. Int J Nephrol Renovasc Dis. 2012. PMID: 22723728 Free PMC article.
-
Contemporary management of phosphorus retention in chronic kidney disease: a review.Clin Exp Nephrol. 2015 Dec;19(6):985-99. doi: 10.1007/s10157-015-1126-y. Epub 2015 Jun 2. Clin Exp Nephrol. 2015. PMID: 26032778 Review.
-
Optimizing the cost-effectiveness of treatment for chronic kidney disease-mineral and bone disorder.Kidney Int Suppl (2011). 2013 Dec;3(5):457-461. doi: 10.1038/kisup.2013.95. Kidney Int Suppl (2011). 2013. PMID: 25019030 Free PMC article. Review.
-
Clinical features of CKD-MBD in Japan: cohort studies and registry.Clin Exp Nephrol. 2017 Mar;21(Suppl 1):9-20. doi: 10.1007/s10157-016-1367-4. Epub 2016 Dec 9. Clin Exp Nephrol. 2017. PMID: 27942882 Review.
References
-
- U.S. Renal Data System: USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2009
-
- Fukuhara S, Yamazaki C, Hayashino Y, Higashi T, Eichleay MA, Akiba T, Akizawa T, Saito A, Port FK, Kurokawa K: The organization and financing of end-stage renal disease treatment in Japan. Int J Health Care Finance Econ 7: 217–231, 2007 - PubMed
-
- Komaba H, Moriwaki K, Kamae I, Fukagawa M: Towards cost-effective strategies for treatment of chronic kidney disease-mineral and bone disorder in Japan. Ther Apher Dial 13[Suppl 1]: S28–S35, 2009 - PubMed
-
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 - PubMed
-
- Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67: 1179–1187, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

