In utero electroporation as a tool for genetic manipulation in vivo to study psychiatric disorders: from genes to circuits and behaviors
- PMID: 21551077
- PMCID: PMC3530425
- DOI: 10.1177/1073858411399925
In utero electroporation as a tool for genetic manipulation in vivo to study psychiatric disorders: from genes to circuits and behaviors
Abstract
Many genetic risk factors for major mental disorders have key roles in brain development. Thus, exploring the roles for these genetic factors for brain development at the molecular, cellular, and neuronal circuit level is crucial for discovering how genetic disturbances affect high brain functions, which ultimately lead to disease pathologies. However, it is a tremendously difficult task, given that most mental disorders have genetic complexities in which many genetic risk factors have multiple roles in different cell types and brain regions over a time-course dependent manner. Furthermore, some genetic risk factors are likely to act epistatically in common molecular pathways. For this reason, a technique for spatial and temporal manipulation of multiple genes is necessary for understanding how genetic disturbances contribute to disease etiology. Here, the authors will review the said technique, in utero electroporation, which investigates the molecular disease pathways in rodent models for major mental disorders. This technique is also useful to examine the effect of genetic risks at the behavioral level. Furthermore, the authors will discuss the recent progress of this technology, such as inducible and cell type-specific targeting, as well as nonepisomal genetic manipulation, which provide further availability of this technique for research on major mental disorders.
Conflict of interest statement
The authors disclosed no conflicts of interests with respect to the authorship and/or publication of this article.
Figures


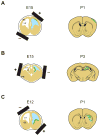

References
-
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6(12):1277–83. - PubMed
-
- Bai J, Ramos RL, Paramasivam M, Siddiqi F, Ackman JB, LoTurco JJ. The role of DCX and LIS1 in migration through the lateral cortical stream of developing forebrain. Dev Neurosci. 2008;30(1–3):144–56. - PubMed
-
- Borrell V, Yoshimura Y, Callaway EM. Targeted gene delivery to telencephalic inhibitory neurons by directional in utero electroporation. J Neurosci Methods. 2005;143(2):151–8. - PubMed
-
- Cetin A, Komai S, Eliava M, Seeburg PH, Osten P. Stereotaxic gene delivery in the rodent brain. Nat Protoc. 2006;1(6):3166–73. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

