Highly fluorescent guanosine mimics for folding and energy transfer studies
- PMID: 21551219
- PMCID: PMC3159459
- DOI: 10.1093/nar/gkr281
Highly fluorescent guanosine mimics for folding and energy transfer studies
Abstract
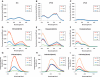
Guanosines with substituents at the 8-position can provide useful fluorescent probes that effectively mimic guanine residues even in highly demanding model systems such as polymorphic G-quadruplexes and duplex DNA. Here, we report the synthesis and photophysical properties of a small family of 8-substituted-2'-deoxyguanosines that have been incorporated into the human telomeric repeat sequence using phosphoramidite chemistry. These include 8-(2-pyridyl)-2'-deoxyguanosine (2PyG), 8-(2-phenylethenyl)-2'-deoxyguanosine (StG) and 8-[2-(pyrid-4-yl)-ethenyl]-2'-deoxyguanosine (4PVG). On DNA folding and stability, 8-substituted guanosines can exhibit context-dependent effects but were better tolerated by G-quadruplex and duplex structures than pyrimidine mismatches. In contrast to previously reported fluorescent guanine analogs, 8-substituted guanosines exhibit similar or even higher quantum yields upon their incorporation into nucleic acids (Φ = 0.02-0.45). We have used these highly emissive probes to quantify energy transfer efficiencies from unmodified DNA nucleobases to 8-substituted guanosines. The resulting DNA-to-probe energy transfer efficiencies (η(t)) are highly structure selective, with η(t)(duplex) < η(t)(single-strand) < η(t)(G-quadruplex). These trends were independent of the exact structural features and thermal stabilities of the G-quadruplexes or duplexes containing them. The combination of efficient energy transfer, high probe quantum yield, and high molar extinction coefficient of the DNA provides a highly sensitive and reliable readout of G-quadruplex formation even in highly diluted sample solutions of 0.25 nM.
Figures


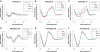
Similar articles
-
Cation-mediated energy transfer in G-quadruplexes revealed by an internal fluorescent probe.J Am Chem Soc. 2010 Dec 29;132(51):18004-7. doi: 10.1021/ja1079578. Epub 2010 Dec 2. J Am Chem Soc. 2010. PMID: 21125997
-
Fluorescence properties of 8-(2-pyridyl)guanine "2PyG" as compared to 2-aminopurine in DNA.Chembiochem. 2011 Sep 5;12(13):2044-51. doi: 10.1002/cbic.201100214. Epub 2011 Jul 22. Chembiochem. 2011. PMID: 21786378
-
Site-specific control of N7-metal coordination in DNA by a fluorescent purine derivative.Chemistry. 2012 Jan 2;18(1):245-54. doi: 10.1002/chem.201102349. Epub 2011 Dec 5. Chemistry. 2012. PMID: 22143992
-
Fluorescent probes for G-quadruplex structures.Chembiochem. 2013 Mar 18;14(5):540-58. doi: 10.1002/cbic.201200612. Epub 2013 Feb 25. Chembiochem. 2013. PMID: 23440895 Review.
-
Fluorescence resonance energy transfer in the studies of guanine quadruplexes.Methods Mol Biol. 2006;335:311-41. doi: 10.1385/1-59745-069-3:311. Methods Mol Biol. 2006. PMID: 16785636 Review.
Cited by
-
Probing the competition between duplex and G-quadruplex/i-motif structures using a conformation-sensitive fluorescent nucleoside probe.Org Biomol Chem. 2018 Jun 6;16(22):4141-4150. doi: 10.1039/c8ob00646f. Org Biomol Chem. 2018. PMID: 29781489 Free PMC article.
-
Fluorescence-based tools to probe G-quadruplexes in cell-free and cellular environments.RSC Adv. 2018;8(45):25673-25694. doi: 10.1039/C8RA03708F. Epub 2018 Jul 17. RSC Adv. 2018. PMID: 30210793 Free PMC article.
-
C-Linked 8-aryl guanine nucleobase adducts: biological outcomes and utility as fluorescent probes.Chem Sci. 2016 Jun 1;7(6):3482-3493. doi: 10.1039/c6sc00053c. Epub 2016 Feb 24. Chem Sci. 2016. PMID: 29997840 Free PMC article. Review.
-
The exception that confirms the rule: a higher-order telomeric G-quadruplex structure more stable in sodium than in potassium.Nucleic Acids Res. 2016 Apr 7;44(6):2926-35. doi: 10.1093/nar/gkw003. Epub 2016 Jan 13. Nucleic Acids Res. 2016. PMID: 26762980 Free PMC article.
-
DNA as UV light-harvesting antenna.Nucleic Acids Res. 2018 Apr 20;46(7):3543-3551. doi: 10.1093/nar/gkx1185. Nucleic Acids Res. 2018. PMID: 29186575 Free PMC article.

