Antiangiogenic antitumor activities of IGFBP-3 are mediated by IGF-independent suppression of Erk1/2 activation and Egr-1-mediated transcriptional events
- PMID: 21551235
- PMCID: PMC3167363
- DOI: 10.1182/blood-2010-08-299784
Antiangiogenic antitumor activities of IGFBP-3 are mediated by IGF-independent suppression of Erk1/2 activation and Egr-1-mediated transcriptional events
Abstract
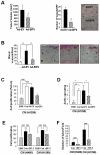
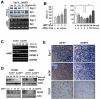
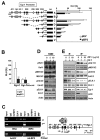
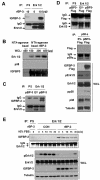
Most antiangiogenic therapies currently being evaluated in clinical trials target the vascular endothelial growth factor pathway; however, the tumor vasculature can acquire resistance to vascular endothelial growth factor-targeted therapy by shifting to other angiogenesis mechanisms. Insulin-like growth factor binding protein-3 (IGFBP-3) has been reported to suppress tumor growth and angiogenesis by both IGF-dependent and IGF-independent mechanisms; however, understanding of its IGF-independent mechanisms is limited. We observed that IGFBP-3 blocked tumor angiogenesis and growth in non-small cell lung cancer and head and neck squamous cell carcinoma. Conditioned media from an IGFBP-3-treated non-small cell lung cancer cell line displayed a significantly decreased capacity to induce HUVEC proliferation and aortic sprouting. In cancer cells, IGFBP-3 directly interacted with Erk1/2, leading to inactivation of Erk1/2 and Elk-1, and suppressed transcription of early growth response protein 1 and its target genes, basic fibroblast growth factor and platelet-derived growth factor. These data suggest that IGF-independent Erk1/2 inactivation and decreased IGFBP-3-induced Egr-1 expression block the autocrine and paracrine loops of angiogenic factors in vascular endothelial and cancer cells. Together, these findings provide a molecular framework of IGFBP-3's IGF-independent antiangiogenic antitumor activities. Future studies are needed for development of IGFBP-3 as a new line of antiangiogengic cancer drug.
Figures



Similar articles
-
Insulin-like growth factor binding protein-3 suppresses vascular endothelial growth factor expression and tumor angiogenesis in head and neck squamous cell carcinoma.Cancer Sci. 2012 Jul;103(7):1259-66. doi: 10.1111/j.1349-7006.2012.02301.x. Epub 2012 May 25. Cancer Sci. 2012. PMID: 22494072 Free PMC article.
-
Identification of insulin-like growth factor binding protein-3 as a farnesyl transferase inhibitor SCH66336-induced negative regulator of angiogenesis in head and neck squamous cell carcinoma.Clin Cancer Res. 2006 Jan 15;12(2):653-61. doi: 10.1158/1078-0432.CCR-05-1725. Clin Cancer Res. 2006. PMID: 16428512
-
SKLB610: a novel potential inhibitor of vascular endothelial growth factor receptor tyrosine kinases inhibits angiogenesis and tumor growth in vivo.Cell Physiol Biochem. 2011;27(5):565-74. doi: 10.1159/000329978. Epub 2011 Jun 15. Cell Physiol Biochem. 2011. PMID: 21691074
-
Targeting angiogenesis in squamous non-small cell lung cancer.Drugs. 2014 Mar;74(4):403-13. doi: 10.1007/s40265-014-0182-z. Drugs. 2014. PMID: 24578213 Free PMC article. Review.
-
[Responses to targeted therapies: lung cancer].Ann Pathol. 2009 Nov;29 Spec No 1:S77-80. doi: 10.1016/j.annpat.2009.07.033. Epub 2009 Oct 8. Ann Pathol. 2009. PMID: 19887261 Review. French. No abstract available.
Cited by
-
miR-132 promotes retinal neovascularization under anoxia and reoxygenation conditions through up-regulating Egr1, ERK2, MMP2, VEGFA and VEGFC expression.Int J Clin Exp Pathol. 2017 Aug 1;10(8):8845-8857. eCollection 2017. Int J Clin Exp Pathol. 2017. PMID: 31966751 Free PMC article.
-
Staphylococcus aureus β-Toxin Exerts Anti-angiogenic Effects by Inhibiting Re-endothelialization and Neovessel Formation.Front Microbiol. 2022 Feb 3;13:840236. doi: 10.3389/fmicb.2022.840236. eCollection 2022. Front Microbiol. 2022. PMID: 35185854 Free PMC article.
-
Transcriptional and posttranslational regulation of insulin-like growth factor binding protein-3 by Akt3.Carcinogenesis. 2014 Oct;35(10):2232-43. doi: 10.1093/carcin/bgu129. Epub 2014 Jun 18. Carcinogenesis. 2014. PMID: 24942865 Free PMC article.
-
Stimulation of Interferon-Stimulated Gene 20 by Thyroid Hormone Enhances Angiogenesis in Liver Cancer.Neoplasia. 2018 Jan;20(1):57-68. doi: 10.1016/j.neo.2017.10.007. Epub 2017 Nov 29. Neoplasia. 2018. PMID: 29195126 Free PMC article.
-
Therapeutic potential of targeting protein for Xklp2 silencing for pancreatic cancer.Cancer Med. 2015 Jul;4(7):1091-100. doi: 10.1002/cam4.453. Epub 2015 Apr 27. Cancer Med. 2015. PMID: 25914189 Free PMC article.
References
-
- van Moorselaar RJ, Voest EE. Angiogenesis in prostate cancer: its role in disease progression and possible therapeutic approaches. Mol Cell Endocrinol. 2002;197(1–2):239–250. - PubMed
-
- Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2(10):795–803. - PubMed
-
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–286. - PubMed
-
- Granata R, Trovato L, Garbarino G, et al. Dual effects of IGFBP-3 on endothelial cell apoptosis and survival: involvement of the sphingolipid signaling pathways. FASEB J. 2004;18(12):1456–1458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

