Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages
- PMID: 21551236
- PMCID: PMC3138686
- DOI: 10.1182/blood-2010-12-327353
Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages
Abstract
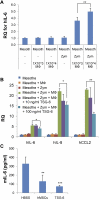
Human mesenchymal stem/progenitor cells (hMSCs) repair tissues and modulate immune systems but the mechanisms are not fully understood. We demonstrated that hMSCs are activated by inflammatory signals to secrete the anti-inflammatory protein, TNF-α-stimulated gene 6 protein (TSG-6) and thereby create a negative feedback loop that reduces inflammation in zymosan-induced peritonitis. The results demonstrate for the first time that TSG-6 interacts through the CD44 receptor on resident macrophages to decrease zymosan/TLR2-mediated nuclear translocation of the NF-κB. The negative feedback loop created by MSCs through TSG-6 attenuates the inflammatory cascade that is initiated by resident macrophages and then amplified by mesothelial cells and probably other cells of the peritoneum. Because inflammation underlies many pathologic processes, including immune responses, the results may explain the beneficial effects of MSCs and TSG-6 in several disease models.
Figures





References
-
- Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267–274. - PubMed
-
- Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. - PubMed
-
- Eaves CJ, Cashman JD, Sutherland HJ, et al. Molecular analysis of primitive hematopoietic cell proliferation control mechanisms. Ann N Y Acad Sci. 1991;628:298–306. - PubMed
-
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. - PubMed
-
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

