Motivating smokers in the hospital pulmonary function laboratory to quit smoking by use of the lung age concept
- PMID: 21551248
- PMCID: PMC3203132
- DOI: 10.1093/ntr/ntr096
Motivating smokers in the hospital pulmonary function laboratory to quit smoking by use of the lung age concept
Abstract
Introduction: The purpose of this study was to investigate the use of lung age to motivate a quit attempt among smokers presenting to a hospital pulmonary function testing (PFT) laboratory.
Methods: Participants were randomized to receive a lung age-based motivational strategy (intervention group) versus standard care (control group). At 1 month, all participants were interviewed by telephone to determine whether they made a quit attempt.
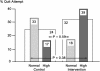
Results: A total of 67 participants were enrolled, and 51 completed the study. Baseline mean data included age = 52 years, 70% women, 40 pack-years of smoking, FEV(1) = 69% predicted, and lung age = 83 years. The quit attempt rates were not different between the intervention and control groups (32% vs. 24%, respectively, p = .59). There was a near significant interaction between lung age and intervention strategy (p = .089), with quit attempt rates among those with normal lung age of 18% in the intervention group versus 33% in the control group and among those with high (worse) lung age of 39% in the intervention group versus 17% in the control group; p = .38.
Conclusions: Using lung age to motivate smokers presenting to the PFT laboratory to quit may succeed in patients with high lung age but may undermine motivation in smokers with normal lung age. Further work is needed to refine the approach to smokers with normal lung age.
Figures
Similar articles
-
The effect of motivational lung age feedback on short-term quit rates in smokers seeking intensive group treatment: A randomized controlled pilot study.Drug Alcohol Depend. 2015 Aug 1;153:271-7. doi: 10.1016/j.drugalcdep.2015.05.007. Epub 2015 May 18. Drug Alcohol Depend. 2015. PMID: 26051163 Free PMC article. Clinical Trial.
-
A Pilot Randomized Clinical Trial of Brief Interventions to Encourage Quit Attempts in Smokers From Socioeconomic Disadvantage.Nicotine Tob Res. 2020 Aug 24;22(9):1500-1508. doi: 10.1093/ntr/ntaa047. Nicotine Tob Res. 2020. PMID: 32161942 Free PMC article. Clinical Trial.
-
Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial.BMJ. 2008 Mar 15;336(7644):598-600. doi: 10.1136/bmj.39503.582396.25. Epub 2008 Mar 6. BMJ. 2008. PMID: 18326503 Free PMC article. Clinical Trial.
-
Immediate and short-term impact of a brief motivational smoking intervention using a biomedical risk assessment: the Get PHIT trial.Nicotine Tob Res. 2009 Apr;11(4):394-403. doi: 10.1093/ntr/ntp004. Epub 2009 Mar 18. Nicotine Tob Res. 2009. PMID: 19299409 Free PMC article. Clinical Trial.
-
Smoking Cessation in the ITALUNG Lung Cancer Screening: What Does "Teachable Moment" Mean?Nicotine Tob Res. 2020 Aug 24;22(9):1484-1491. doi: 10.1093/ntr/ntz148. Nicotine Tob Res. 2020. PMID: 31504798 Clinical Trial.
Cited by
-
Codesigning a Digital Type 2 Diabetes Risk Communication Tool in Singapore: Qualitative Participatory Action Research Approach.JMIR Form Res. 2024 Nov 5;8:e50456. doi: 10.2196/50456. JMIR Form Res. 2024. PMID: 39500495 Free PMC article.
-
Biomedical risk assessment as an aid for smoking cessation.Cochrane Database Syst Rev. 2019 Mar 26;3(3):CD004705. doi: 10.1002/14651858.CD004705.pub5. Cochrane Database Syst Rev. 2019. PMID: 30912847 Free PMC article.
-
Comparative Analysis of Three Different Drug Distribution Schemes for Smoking Cessation.Turk Thorac J. 2021 Mar;22(2):110-117. doi: 10.5152/TurkThoracJ.2020.19111. Epub 2021 Mar 1. Turk Thorac J. 2021. PMID: 33871333 Free PMC article.
-
Self-Reported Smoking Status 10-Months After a Single Session Intervention Including an Education Conference About Smoking Harms and Announcement of Spirometric Lung-Age.Clin Med Insights Circ Respir Pulm Med. 2021 Oct 20;15:11795484211047041. doi: 10.1177/11795484211047041. eCollection 2021. Clin Med Insights Circ Respir Pulm Med. 2021. PMID: 34690503 Free PMC article.
-
The effect of motivational lung age feedback on short-term quit rates in smokers seeking intensive group treatment: A randomized controlled pilot study.Drug Alcohol Depend. 2015 Aug 1;153:271-7. doi: 10.1016/j.drugalcdep.2015.05.007. Epub 2015 May 18. Drug Alcohol Depend. 2015. PMID: 26051163 Free PMC article. Clinical Trial.
References
-
- Anthonisen N, Skeans M, Wise R, Manfeda J, Kanner R, Connett J, et al. The effects of smoking cessation intervention on 14.5-year mortality. Annals of Internal Medicine. 2005;142:233–239. doi:10.1016/j.accreview.2005.05.034. - PubMed
-
- Bize R, Burnand B, Mueller Y, Rege W, Cornuz J. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database of Systematic Reviews. 2009;(2) CD004705.pub2. doi:10.1002/14651858.CD004705.pub3. - PubMed
-
- Bohadana A, Nilsson F, Martinet Y. Detecting airflow obstruction in smoking cessation trials. A rationale for routine spirometry. Chest. 2005;128:1252–1257. doi:10.1378/chest.128.3.1252. - PubMed
-
- Borrelli B, McQuaid E, Becker B, Hammond K, Papandonatos G, Fritz G, et al. Motivating parents of kids with asthma to quit smoking: The PAQS project. Health Education Research. 2002;17:659–669. doi:10.1093/her/17.5.659. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

