Regulation of the Edwardsiella ictaluri type III secretion system by pH and phosphate concentration through EsrA, EsrB, and EsrC
- PMID: 21551284
- PMCID: PMC3127708
- DOI: 10.1128/AEM.00195-11
Regulation of the Edwardsiella ictaluri type III secretion system by pH and phosphate concentration through EsrA, EsrB, and EsrC
Abstract
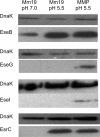
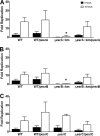
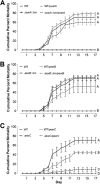
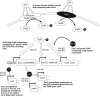
A recently described Edwardsiella ictaluri type III secretion system (T3SS) with functional similarity to the Salmonella pathogenicity island 2 T3SS is required for replication in channel catfish head-kidney-derived macrophages (HKDM) and virulence in channel catfish. Quantitative PCR and Western blotting identified low pH and phosphate limitation as conducive to expression of the E. ictaluri T3SS, growth conditions that mimic the phagosomal environment. Mutagenesis studies demonstrated that expression is under the control of the EsrAB two-component regulatory system. EsrB also induces upregulation of the AraC-type regulatory protein EsrC, which enhances expression of the EscB/EseG chaperone/effector operon in concert with EsrB and induces expression of the pEI1-encoded effector, EseH. EsrC also induces expression of a putative type VI secretion system translocon protein, EvpC, which is secreted under the same low-pH conditions as the T3SS translocon proteins. The pEI2-encoded effector, EseI, was upregulated under low-pH and low-phosphate conditions but not in an EsrB- or EsrC-dependent manner. Mutations of EsrA and EsrB both resulted in loss of the ability to replicate in HKDM and full attenuation in the channel catfish host. Mutation of EsrC did not affect intracellular replication but did result in attenuation in catfish. Although EsrB is the primary transcriptional regulator for E. ictaluri genes within the T3SS pathogenicity island, EsrC regulates expression of the plasmid-carried effector eseH and appears to mediate coordinated expression of the T6SS with the T3SS.
Figures

References
-
- Anderson D. M., Schneewind O. 1997. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 278:1140–1143 - PubMed
-
- Anonymous 2003. Catfish 2003. I. Reference of fingerling catfish health and production practices in the United States. N406.1103. USDA, APHIS, VS, CEAH, National Animal Health Monitoring System, Fort Collins, CO: http://www.aphis.usda.gov/animal_health/nahms/aquaculture/downloads /cat...
-
- Anonymous 2003. Catfish 2003. II. Reference of foodsize catfish health and production practices in the United States. N406.1103. USDA, APHIS, VS, CEAH, National Animal Health Monitoring System, Fort Collins, CO: http://www.aphis.usda.gov/animal_health/nahms/aquaculture/downloads /cat...
-
- Ausubel F. M., et al. 1994. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY
-
- Beuzon C. R., Banks G., Deiwick J., Hensel M., Holden D. W. 1999. pH-dependent secretion of SseB, a product of the SPI-2 type III secretion system of Salmonella typhimurium. Mol. Microbiol. 33:806–816 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

