HIV-1 reduces Abeta-degrading enzymatic activities in primary human mononuclear phagocytes
- PMID: 21551363
- PMCID: PMC3110566
- DOI: 10.4049/jimmunol.1100211
HIV-1 reduces Abeta-degrading enzymatic activities in primary human mononuclear phagocytes
Abstract
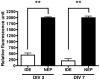
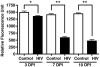
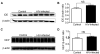
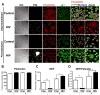
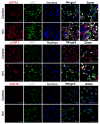
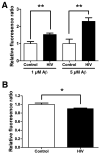
The advent and wide introduction of antiretroviral therapy has greatly improved the survival and longevity of HIV-infected patients. Unfortunately, despite antiretroviral therapy treatment, these patients are still afflicted with many complications including cognitive dysfunction. There is a growing body of reports indicating accelerated deposition of amyloid plaques, which are composed of amyloid-β peptide (Aβ), in HIV-infected brains, though how HIV viral infection precipitates Aβ accumulation is poorly understood. It is suggested that viral infection leads to increased production and impaired degradation of Aβ. Mononuclear phagocytes (macrophages and microglia) that are productively infected by HIV in brains play a pivotal role in Aβ degradation through the expression and execution of two endopeptidases, neprilysin (NEP) and insulin-degrading enzyme. In this study, we report that NEP has the dominant endopeptidase activity toward Aβ in macrophages. Further, we demonstrate that monomeric Aβ degradation by primary cultured macrophages and microglia was significantly impaired by HIV infection. This was accompanied with great reduction of NEP endopeptidase activity, which might be due to the diminished transport of NEP to the cell surface and intracellular accumulation at the endoplasmic reticulum and lysosomes. Therefore, these data suggest that malfunction of NEP in infected macrophages may contribute to acceleration of β amyloidosis in HIV-inflicted brains, and modulation of macrophages may be a potential preventative target of Aβ-related cognitive disorders in HIV-affected patients.
Figures


Similar articles
-
Insulin-signaling Pathway Regulates the Degradation of Amyloid β-protein via Astrocytes.Neuroscience. 2018 Aug 10;385:227-236. doi: 10.1016/j.neuroscience.2018.06.018. Epub 2018 Jun 20. Neuroscience. 2018. PMID: 29932983
-
HIV-1 Tat inhibits neprilysin and elevates amyloid beta.AIDS. 2005 Jan 28;19(2):127-35. doi: 10.1097/00002030-200501280-00004. AIDS. 2005. PMID: 15668537
-
Aβ-degrading enzymes: potential for treatment of Alzheimer disease.J Neuropathol Exp Neurol. 2011 Nov;70(11):944-59. doi: 10.1097/NEN.0b013e3182345e46. J Neuropathol Exp Neurol. 2011. PMID: 22002425 Review.
-
HIV-1 increases extracellular amyloid-beta levels through neprilysin regulation in primary cultures of human astrocytes.J Cell Physiol. 2019 May;234(5):5880-5887. doi: 10.1002/jcp.26462. Epub 2018 Dec 7. J Cell Physiol. 2019. PMID: 29323711
-
Beta-amyloid catabolism: roles for neprilysin (NEP) and other metallopeptidases?J Neurochem. 2002 Apr;81(1):1-8. doi: 10.1046/j.1471-4159.2002.00855.x. J Neurochem. 2002. PMID: 12067222 Review.
Cited by
-
Infectious agents and neurodegeneration.Mol Neurobiol. 2012 Dec;46(3):614-38. doi: 10.1007/s12035-012-8320-7. Epub 2012 Aug 17. Mol Neurobiol. 2012. PMID: 22899188 Free PMC article. Review.
-
Blood amyloid-β protein isoforms are affected by HIV-1 in a subtype-dependent pattern.J Neurovirol. 2020 Feb;26(1):3-13. doi: 10.1007/s13365-019-00783-6. Epub 2019 Jul 7. J Neurovirol. 2020. PMID: 31281948 Free PMC article.
-
Biomarkers of neuronal injury and amyloid metabolism in the cerebrospinal fluid of patients infected with HIV-1 subtypes B and C.J Neurovirol. 2018 Feb;24(1):28-40. doi: 10.1007/s13365-017-0591-3. Epub 2017 Oct 23. J Neurovirol. 2018. PMID: 29063514 Free PMC article.
-
FGF2 gene transfer restores hippocampal functions in mouse models of Alzheimer's disease and has therapeutic implications for neurocognitive disorders.Proc Natl Acad Sci U S A. 2011 Dec 6;108(49):E1339-48. doi: 10.1073/pnas.1102349108. Epub 2011 Oct 31. Proc Natl Acad Sci U S A. 2011. PMID: 22042871 Free PMC article.
-
High glucose enhances HIV entry into T cells through upregulation of CXCR4.J Leukoc Biol. 2013 Oct;94(4):769-77. doi: 10.1189/jlb.0313142. Epub 2013 Aug 2. J Leukoc Biol. 2013. PMID: 23911867 Free PMC article.
References
-
- Pulliam L. HIV regulation of amyloid beta production. J Neuroimmune Pharmacol. 2009;4:213–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

