Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer
- PMID: 21551365
- PMCID: PMC3110549
- DOI: 10.4049/jimmunol.1100274
Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer
Abstract
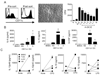
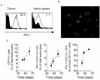
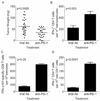
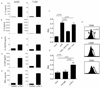
Within the ovarian cancer microenvironment, there are several mechanisms that suppress the actions of antitumor immune effectors. Delineating the complex immune microenvironment is an important goal toward developing effective immune-based therapies. A dominant pathway of immune suppression in ovarian cancer involves tumor-associated and dendritic cell (DC)-associated B7-H1. The interaction of B7-H1 with PD-1 on tumor-infiltrating T cells is a widely cited theory of immune suppression involving B7-H1 in ovarian cancer. Recent studies suggest that the B7-H1 ligand, programmed death receptor-1 (PD-1), is also expressed on myeloid cells, complicating interpretations of how B7-H1 regulates DC function in the tumor. In this study, we found that ovarian cancer-infiltrating DCs progressively expressed increased levels of PD-1 over time in addition to B7-H1. These dual-positive PD-1(+) B7-H1(+) DCs have a classical DC phenotype (i.e., CD11c(+)CD11b(+)CD8(-)), but are immature, suppressive, and respond poorly to danger signals. Accumulation of PD-1(+)B7-H1(+) DCs in the tumor was associated with suppression of T cell activity and decreased infiltrating T cells in advancing tumors. T cell suppressor function of these DCs appeared to be mediated by T cell-associated PD-1. In contrast, ligation of PD-1 expressed on the tumor-associated DCs suppressed NF-κB activation, release of immune regulatory cytokines, and upregulation of costimulatory molecules. PD-1 blockade in mice bearing ovarian cancer substantially reduced tumor burden and increased effector Ag-specific T cell responses. Our results reveal a novel role of tumor infiltrating PD-1(+)B7-H1(+) DCs in mediating immune suppression in ovarian cancer.
Conflict of interest statement
Figures


References
-
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003;348:203–213. - PubMed
-
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. U S A. 2005;102:18538–18543. - PMC - PubMed
-
- Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, Pastan I. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin. Cancer Res. 2005;11:3814–3820. - PubMed
-
- Goodell V, Salazar LG, Urban N, Drescher CW, Gray H, Swensen RE, McIntosh MW, Disis ML. Antibody immunity to the p53 oncogenic protein is a prognostic indicator in ovarian cancer. J. Clin. Oncol. 2006;24:762–768. - PubMed
-
- Knutson KL, Krco CJ, Erskine CL, Goodman K, Kelemen LE, Wettstein PJ, Low PS, Hartmann LC, Kalli KR. T-cell immunity to the folate receptor alpha is prevalent in women with breast or ovarian cancer. J. Clin. Oncol. 2006;24:4254–4261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

