Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy
- PMID: 21551414
- PMCID: PMC3175954
- DOI: 10.1167/iovs.11-7357
Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy
Abstract
Purpose: This study tested a hypothesis that tissue-selective targeted decorin gene therapy delivered to the stroma with adeno-associated virus serotype 5 (AAV5) inhibits corneal fibrosis in vivo without significant side effects.
Methods: An in vivo rabbit model of corneal fibrosis was used. Targeted decorin gene therapy was delivered to the rabbit cornea by a single topical application of AAV5 (100 μL; 6.5 × 10(12) μg/mL) onto the bare stroma for 2 minutes. The levels of corneal fibrosis were determined with stereomicroscopy, slit lamp biomicroscopy, α-smooth muscle actin (αSMA), fibronectin, and F-actin immunocytochemistry, and/or immunoblotting. CD11b, F4/80 immunocytochemistry, and TUNEL assay were used to examine immunogenicity and cytotoxicity of AAV5 to the cornea. Transmission electron microscopy (TEM) was used to investigate ultrastructural features. Slot-blot-quantified the copy number of AAV5-delivered decorin genes.
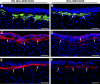
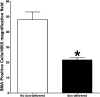
Results: Selective decorin delivery into the stroma showed a significant (P < 0.01) decrease in corneal haze (1.3 ± 0.3) compared with the no-decorin-delivered control rabbit corneas (3 ± 0.4) quantified using slit lamp biomicroscopy. Immunostaining and immunoblot analyses detected significantly reduced levels of αSMA, F-actin, and fibronectin proteins (59%-73%; P < 0.001 or <0.01) in decorin-delivered rabbit corneas compared with the no-decorin-delivered controls. The visual clinical eye examination, slit lamp clinical studies, TUNEL, CD11b, and F4/80 assays revealed that AAV5-mediated decorin gene therapy is nonimmunogenic and nontoxic for the cornea. TEM studies suggested that decorin gene delivery does not jeopardize collagen fibrillogenesis as no significant differences in collagen fibril diameter and arrangement were observed in decorin-delivered and no-decorin-delivered control corneas.
Conclusions: Tissue-targeted AAV5-mediated decorin gene therapy is effective and safe for treating corneal fibrosis in vivo.
Figures






References
-
- Foster A, Resnikoff S. The impact of Vision 2020 on global blindness. Eye. 2005;19:1133–1135 - PubMed
-
- Congdon N, O'Colmain B, Klaver CC, et al. Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485 - PubMed
-
- Wilson SE, Mohan RR, Mohan RR, Ambrósio R, Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20:625–637 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

