Genetic analysis of basophil function in vivo
- PMID: 21552267
- PMCID: PMC3271435
- DOI: 10.1038/ni.2036
Genetic analysis of basophil function in vivo
Erratum in
- Nat Immunol. 2011 Jun;12(6):3 p following 535
Abstract
Contributions by basophils to allergic and helminth immunity remain incompletely defined. Using sensitive interleukin 4 (Il4) reporter alleles, we demonstrate here that basophil IL-4 production occurs by a CD4(+) T cell-dependent process restricted to the peripheral tissues affected. We genetically marked and achieved specific deletion of basophils and found that basophils did not mediate T helper type 2 (T(H)2) priming in vivo. Two-photon imaging confirmed that basophils did not interact with antigen-specific T cells in lymph nodes but engaged in prolonged serial interactions with T cells in lung tissues. Although targeted deletion of IL-4 and IL-13 in either CD4(+) T cells or basophils had a minimal effect on worm clearance, deletion from both lineages demonstrated a nonredundant role for basophil cytokines in primary helminth immunity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

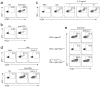
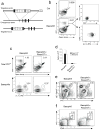
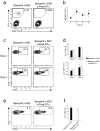
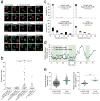
References
-
- Chan MS. The global burden of intestinal nematode infections--fifty years on. Parasitol Today. 1997;13:438–443. - PubMed
-
- Urban JF, Jr, Maliszewski CR, Madden KB, Katona IM, Finkelman FD. IL-4 treatment can cure established gastrointestinal nematode infections in immunocompetent and immunodeficient mice. J Immunol. 1995;154:4675–4684. - PubMed
-
- Urban JF, Jr, et al. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

