Number needed to treat and number needed to harm with paliperidone palmitate relative to long-acting haloperidol, bromperidol, and fluphenazine decanoate for treatment of patients with schizophrenia
- PMID: 21552311
- PMCID: PMC3083982
- DOI: 10.2147/NDT.S17177
Number needed to treat and number needed to harm with paliperidone palmitate relative to long-acting haloperidol, bromperidol, and fluphenazine decanoate for treatment of patients with schizophrenia
Abstract
Background: We analyzed data retrieved through a PubMed search of randomized, placebo-controlled trials of first-generation antipsychotic long-acting injectables (haloperidol decanoate, bromperidol decanoate, and fluphenazine decanoate), and a company database of paliperidone palmitate, to compare the benefit-risk ratio in patients with schizophrenia.
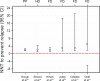
Methods: From the eight studies that met our selection criteria, two efficacy and six safety parameters were selected for calculation of number needed to treat (NNT), number needed to harm (NNH), and the likelihood of being helped or harmed (LHH) using comparisons of active drug relative to placebo. NNTs for prevention of relapse ranged from 2 to 5 for paliperidone palmitate, haloperidol decanoate, and fluphenazine decanoate, indicating a moderate to large effect size.
Results: Among the selected maintenance studies, NNH varied considerably, but indicated a lower likelihood of encountering extrapyramidal side effects, such as akathisia, tremor, and tardive dyskinesia, with paliperidone palmitate versus placebo than with first-generation antipsychotic depot agents versus placebo. This was further supported by an overall higher NNH for paliperidone palmitate versus placebo with respect to anticholinergic use and Abnormal Involuntary Movement Scale positive score. LHH for preventing relapse versus use of anticholinergics was 15 for paliperidone palmitate and 3 for fluphenazine decanoate, favoring paliperidone palmitate.
Conclusion: Overall, paliperidone palmitate had a similar NNT and a more favorable NNH compared with the first-generation long-acting injectables assessed.
Keywords: first-generation antipsychotics; long-acting injectables; number needed to harm; number needed to treat; paliperidone palmitate; randomized; second-generation antipsychotics.
Figures
References
-
- Nayak RK, Doose DR, Nair NP. The bioavailability and pharmacokinetics of oral and depot intramuscular haloperidol in schizophrenic patients. J Clin Pharmacol. 1987;27(2):144–150. - PubMed
-
- Olivares JM, Rodriguez-Morales A, Diels J, et al. Long-term outcomes in patients with schizophrenia treated with risperidone long-acting injection or oral antipsychotics in Spain: Results from the electronic Schizophrenia Treatment Adherence Registry (e-STAR) Eur Psychiatry. 2009;24(5):287–296. - PubMed
-
- Bransgrove LL, Kelly MW. Movement disorders in patients treated with long-acting injectable antipsychotic drugs. Am J Hosp Pharm. 1994;51(7):895–899. - PubMed
-
- Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: A systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414–425. - PubMed
-
- Gharabawi GM, Bossie CA, Zhu Y, et al. An assessment of emergent tardive dyskinesia and existing dyskinesia in patients receiving long-acting, injectable risperidone: Results from a long-term study. Schizophr Res. 2005;77(2–3):129–139. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

