N,N,N-Trimethyl chitosan nanoparticles for the delivery of monoclonal antibodies against hepatocellular carcinoma cells
- PMID: 21552341
- PMCID: PMC3088426
- DOI: 10.1016/j.carbpol.2011.02.018
N,N,N-Trimethyl chitosan nanoparticles for the delivery of monoclonal antibodies against hepatocellular carcinoma cells
Abstract
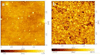
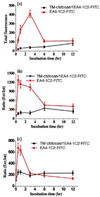
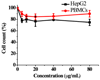
N,N,N-Trimethyl chitosan chloride is capable of forming nanocomplexes with protein through ionotropic gelation. A monoclonal antibody, raised against human liver heparan sulfate proteoglycan and specifically inhibiting hepatocellular carcinoma in vitro, was prepared in nanocomplexes of this modified chitosan. The smallest nanocomplexes (59 ± 17 nm, zeta-potential 16.5 ± 0.5 mV) were obtained at polysaccharide:antibody ratios of 5:0.3. Spherical particles with a smooth surface and compact structure having a mean diameter of ~11.2 ± 0.09 nm were investigated by Atomic Force Microscopy. Cellular uptake of fluorescently labeled nanocomplexes was studied in mouse monocyte models of cancer and normal cells. External and internal fluorescence was analyzed by flow cytometry. The results demonstrate that the nanocomplexes could enter cells and were retained for a longer period of time in cancer cells where they exhibited greater toxicity. These nanocomplexes appear safe and could potentially enhance the half-life of added antibodies.
Figures

References
-
- Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Advanced Drug Delivery Reviews. 2010;62:59–82. - PubMed
-
- Bao GQ, Li Y, Ma QJ, He XL, Xing JL, Yang XM, Chen ZN. Isolating human antibody against human hepatocellular carcinoma by guided selection. Cancer Biology & Therapy. 2005;4:1374–1380. - PubMed
-
- Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609–1619. - PubMed
-
- Berger J, Reist M, Chenite A, Felt-Baeyens O, Mayer JM, Gurny R. Pseudo-thermosetting chitosan hydrogels for biomedical application. International Journal of Pharmaceutics. 2005;288:17–25. - PubMed
-
- Biswas S, Bhattacharya SC, Moulik SP. Quenching of fluorescence of 1-hydroxypyrene-3,6,8-trisulfonate (HPTS) by Cu2+, Co2+, Ni2+, I−, and cetylpyridinium (CP+) ions in water/AOT/heptane microemulsion. Journal of Colloidal Interface Science. 2004;271:157–162. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

