Quantifying transduction efficiencies of unmodified and tyrosine capsid mutant AAV vectors in vitro using two ocular cell lines
- PMID: 21552473
- PMCID: PMC3087449
Quantifying transduction efficiencies of unmodified and tyrosine capsid mutant AAV vectors in vitro using two ocular cell lines
Abstract
Purpose: With the increasing number of retinal gene-based therapies and therapeutic constructs, in vitro bioassays characterizing vector transduction efficiency and quality are becoming increasingly important. Currently, in vitro assays quantifying vector transduction efficiency are performed predominantly for non-ocular tissues. A human retinal pigment epithelial cell line (ARPE19) and a mouse cone photoreceptor cell line, 661W, have been well characterized and are used for many retinal metabolism and biologic pathway studies. The purpose of this study is to quantify transduction efficiencies of a variety of self-complementary (sc) adeno-associated virus (AAV) vectors in these biologically relevant ocular cell lines using high-throughput fluorescence-activated cell sorting (FACS) analysis.
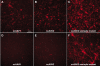
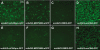
Methods: ARPE19 and 661W cells were infected with sc-smCBA-mCherry packaged in unmodified AAV capsids or capsids containing single/multiple tyrosine-phenylalanine (Y-F) mutations at multiplicity of infections (MOIs) ranging from 100 to 10,000. Three days post infection fluorescent images verified mCherry expression. Following microscopy, FACS analysis was performed to quantify the number of positive cells and the mean intensity of mCherry fluorescence, the product of which is reported as transduction efficiency for each vector. The scAAV vectors containing cone-specific (sc-mCARpro-green fluorescent protein [GFP]), rod-specific (sc-MOPS500-eGFP), retinal pigment epithelium (RPE)-specific (sc-VMD2-GFP), or ubiquitous (sc-smCBA-GFP) promoters were used to infect both cell lines at an MOI of 10,000. Three days post infection, cells were immunostained with an antibody raised against GFP and imaged. Finally, based on our in vitro results, we tested a prediction of transduction efficiency in vivo.
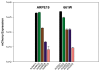
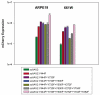
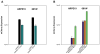
Results: Expression from unmodified scAAV1, scAAV2, scAAV5, and scAAV8 vectors was detectable by FACS in both ARPE19 and 661W cells, with scAAV1 and scAAV2 being the most efficient in both cell lines. scAAV5 showed moderate efficiency in both ARPE19 and 661W cells. scAAV8 was moderately efficient in 661W cells and was by comparison less so in ARPE19 cells; however, transduction was still apparent. scAAV9 performed poorly in both cell types. With some exceptions, the Y-F capsid mutations generally increased the efficiency of scAAV vector transduction, with the increasing number of mutated residues improving efficiency. Results for single scAAV1 and scAAV8 capsid mutants were mixed. In some cases, efficiency improved; in others, it was unchanged or marginally reduced. Retinal-specific promoters were also active in both cell lines, with the 661W cells showing a pattern consistent with the in vivo activity of the respective promoters tested. The prediction based on in vitro data that AAV2 sextuple Y-F mutants would show higher transduction efficiency in RPE relative to AAV2 triple Y-F capsid mutants was validated by evaluating the transduction characteristics of the two mutant vectors in mouse retina.
Conclusions: Our results suggest that this rapid and quantifiable cell-based assay using two biologically relevant ocular cell lines will prove useful in screening and optimizing AAV vectors for application in retina-targeted gene therapies.
Figures

References
-
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9. - PubMed
-
- Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, Flotte TR, Fishman GA, Heon E, Stone EM, Byrne BJ, Jacobson SG, Hauswirth WW. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA. 2008;105:15112–7. - PMC - PubMed
-
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short term results of a phase I trial. Hum Gene Ther. 2008;19:979–90. - PMC - PubMed
-
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell'Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–8. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
