Degeneration of the mouse retina upon dysregulated activity of serum response factor
- PMID: 21552476
- PMCID: PMC3087454
Degeneration of the mouse retina upon dysregulated activity of serum response factor
Abstract
Purpose: Our aim was to generate and phenotypically characterize a transgenic mouse line expressing a constitutively active variant of the transcription regulatory protein serum response factor (SRF), namely the SRF-VP16 protein. This new mouse strain has been registered under the designation Gt(ROSA)26Sor(tm1(SRF-VP16)Antu). We found phenotypic changes upon ectopic expression of SRF-VP16, especially in the mouse retina.
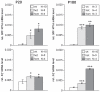
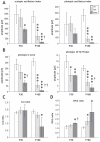
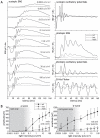
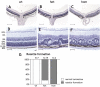
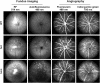
Methods: Using homologous recombination, we integrated an SRF-VP16 conditional (i.e., "flox-STOP" repressed) expression transgene into the Rosa26 locus of murine embryonic stem (ES) cells. These engineered ES cells were used to derive the Gt(ROSA)26Sor(tm1(SRF-VP16)Antu) mouse strain. Semiquantitative real-time PCR was used to determine expression of the SRF-VP16 transgene at the mRNA level, both in young (P20 and P30) and adult (six months old) Gt(ROSA)26Sor(tm1(SRF-VP16)Antu) mice. We also investigated the transcript levels of endogenous Srf and several SRF target genes. Retinal function was tested by electroretinography in both young and adult mice. Morphological abnormalities could be visualized by hematoxylin and eosin staining of sectioned, paraffin-embedded eye tissue samples. Scanning-laser ophthalmoscopy was used to investigate retinal vascularization and degeneration in adult mice.
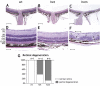
Results: We show that the SRF-VP16 mRNA is expressed to a low but significant degree in the retinas of young and adult animals of the Gt(ROSA)26Sor(tm1(SRF-VP16)Antu) mouse strain, even in the absence of Cre-mediated deletion of the "flox-STOP" cassette. In the retinas of these transgenic mice, endogenous Srf displays elevated transcript levels. Ectopic retinal expression of constitutively active SRF-VP16 is correlated with the malfunction of retinal neurons in both heterozygous and homozygous animals of both age groups (P20 and adult). Additionally, mislamination of retinal cell layers and cellular rosette formations are found in retinas of both heterozygous and homozygous animals of young age. In homozygous individuals, however, the cellular rosettes are more widespread over the fundus. At adult age, retinas both from animals that are heterozygous and homozygous for the floxSTOP/SRF-VP16 transgene display severe degeneration, mainly of the photoreceptor cell layer. Wild-type age-matched littermates, however, do not show any degeneration. The severity of the observed effects correlates with dosage of the transgene.
Conclusions: This is the first report suggesting an influence of the transcription factor SRF on the development and function of the murine retina. Ectopic SRF-VP16 mRNA expression in the retinas of young animals is correlated with photoreceptor layer mislamination and impaired retinal function. At an advanced age of six months, degenerative processes are detected in SRF-VP16 transgenic retinas accompanied by impaired retinal function. The Gt(ROSA)26Sor(tm1(SRF-VP16)Antu) mouse strain represents a genetic SRF gain-of-function mouse model that will complement the current SRF loss-of-function models. It promises to provide new insight into the hitherto poorly defined role of SRF in retinal development and function, including potential contributions to ophthalmologic disorders. Furthermore, using conditional Cre-mediated activation of SRF-VP16, the described mouse strain will enable assessment of the impact of dysregulated SRF activity on the physiologic functions of various other organs.
Figures


References
-
- Graw J. Genetic aspects of embryonic eye development in vertebrates. Dev Genet. 1996;18:181–97. - PubMed
-
- Jean D, Ewan K, Gruss P. Molecular regulators involved in vertebrate eye development. Mech Dev. 1998;76:3–18. - PubMed
-
- Rossant J, Tam PPL, editors. Mouse development: Patterning, morphogenesis, and organogenesis. 1st edition. San Diego: Academic Press; 2002.
-
- Etkin A, Alarcón JM, Weisberg SP, Touzani K, Huang YY, Nordheim A, Kandel ER. A role in learning for SRF: deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron. 2006;50:127–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
