αA-crystallin peptide SDRDKFVIFLDVKHF accumulating in aging lens impairs the function of α-crystallin and induces lens protein aggregation
- PMID: 21552534
- PMCID: PMC3084282
- DOI: 10.1371/journal.pone.0019291
αA-crystallin peptide SDRDKFVIFLDVKHF accumulating in aging lens impairs the function of α-crystallin and induces lens protein aggregation
Abstract
Background: The eye lens is composed of fiber cells that are filled with α-, β- and γ-crystallins. The primary function of crystallins is to maintain the clarity of the lens through ordered interactions as well as through the chaperone-like function of α-crystallin. With aging, the chaperone function of α-crystallin decreases, with the concomitant accumulation of water-insoluble, light-scattering oligomers and crystallin-derived peptides. The role of crystallin-derived peptides in age-related lens protein aggregation and insolubilization is not understood.
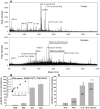
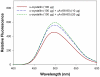
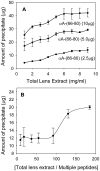
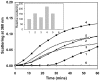
Methodology/principal findings: We found that αA-crystallin-derived peptide, (66)SDRDKFVIFLDVKHF(80), which accumulates in the aging lens, can inhibit the chaperone activity of α-crystallin and cause aggregation and precipitation of lens crystallins. Age-related change in the concentration of αA-(66-80) peptide was estimated by mass spectrometry. The interaction of the peptide with native crystallin was studied by multi-angle light scattering and fluorescence methods. High molar ratios of peptide-to-crystallin were favourable for aggregation and precipitation. Time-lapse recordings showed that, in the presence of αA-(66-80) peptide, α-crystallin aggregates and functions as a nucleus for protein aggregation, attracting aggregation of additional α-, β- and γ-crystallins. Additionally, the αA-(66-80) peptide shares the principal properties of amyloid peptides, such as β-sheet structure and fibril formation.
Conclusions/significance: These results suggest that crystallin-derived peptides such as αA-(66-80), generated in vivo, can induce age-related lens changes by disrupting the structure and organization of crystallins, leading to their insolubilization. The accumulation of such peptides in aging lenses may explain a novel mechanism for age-related crystallin aggregation and cataractogenesis.
Conflict of interest statement
Figures



References
-
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, et al. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. - PubMed
-
- Horwitz J, Emmons T, Takemoto L. The ability of lens alpha crystallin to protect against heat-induced aggregation is age-dependent. Curr Eye Res. 1992;11:817–822. - PubMed
-
- Bron AJ, Vrensen GF, Koretz J, Maraini G, Harding JJ. The ageing lens. Ophthalmologica. 2000;214:86–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

