Genetic analysis reveals an unexpected role of BMP7 in initiation of ureteric bud outgrowth in mouse embryos
- PMID: 21552539
- PMCID: PMC3084290
- DOI: 10.1371/journal.pone.0019370
Genetic analysis reveals an unexpected role of BMP7 in initiation of ureteric bud outgrowth in mouse embryos
Abstract
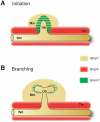
Background: Genetic analysis in the mouse revealed that GREMLIN1 (GREM1)-mediated antagonism of BMP4 is essential for ureteric epithelial branching as the disruption of ureteric bud outgrowth and renal agenesis in Grem1-deficient embryos is restored by additional inactivation of one Bmp4 allele. Another BMP ligand, BMP7, was shown to control the proliferative expansion of nephrogenic progenitors and its requirement for nephrogenesis can be genetically substituted by Bmp4. Therefore, we investigated whether BMP7 in turn also participates in inhibiting ureteric bud outgrowth during the initiation of metanephric kidney development.
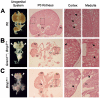
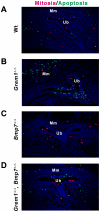
Methodology/principal findings: Genetic inactivation of one Bmp7 allele in Grem1-deficient mouse embryos does not alleviate the bilateral renal agenesis, while complete inactivation of Bmp7 restores ureteric bud outgrowth and branching. In mouse embryos lacking both Grem1 and Bmp7, GDNF/WNT11 feedback signaling and the expression of the Etv4 target gene, which regulates formation of the invading ureteric bud tip, are restored. In contrast to the restoration of ureteric bud outgrowth and branching, nephrogenesis remains aberrant as revealed by the premature loss of Six2 expressing nephrogenic progenitor cells. Therefore, very few nephrons develop in kidneys lacking both Grem1 and Bmp7 and the resulting dysplastic phenotype is indistinguishable from the one of Bmp7-deficient mouse embryos.
Conclusions/significance: Our study reveals an unexpected inhibitory role of BMP7 during the onset of ureteric bud outgrowth. As BMP4, BMP7 and GREM1 are expressed in distinct mesenchymal and epithelial domains, the localized antagonistic interactions of GREM1 with BMPs could restrict and guide ureteric bud outgrowth and branching. The robustness and likely significant redundancy of the underlying signaling system is evidenced by the fact that global reduction of Bmp4 or inactivation of Bmp7 are both able to restore ureteric bud outgrowth and epithelial branching in Grem1-deficient mouse embryos.
Conflict of interest statement
Figures



References
-
- Affolter M, Zeller R, Caussinus E. Tissue remodelling through branching morphogenesis. Nat Rev Mol Cell Biol. 2009;10:831–842. - PubMed
-
- Shakya R, Jho EH, Kotka P, Wu Z, Kholodilov N, et al. The role of GDNF in patterning the excretory system. Dev Biol. 2005;283:70–84. - PubMed
-
- Michos O, Goncalves A, Lopez-Rios J, Tiecke E, Naillat F, et al. Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development. 2007;134:2397–2405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

