Rising from the sea: correlations between sulfated polysaccharides and salinity in plants
- PMID: 21552557
- PMCID: PMC3084243
- DOI: 10.1371/journal.pone.0018862
Rising from the sea: correlations between sulfated polysaccharides and salinity in plants
Abstract
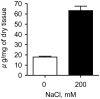
High salinity soils inhibit crop production worldwide and represent a serious agricultural problem. To meet our ever-increasing demand for food, it is essential to understand and engineer salt-resistant crops. In this study, we evaluated the occurrence and function of sulfated polysaccharides in plants. Although ubiquitously present in marine algae, the presence of sulfated polysaccharides among the species tested was restricted to halophytes, suggesting a possible correlation with salt stress or resistance. To test this hypothesis, sulfated polysaccharides from plants artificially and naturally exposed to different salinities were analyzed. Our results revealed that the sulfated polysaccharide concentration, as well as the degree to which these compounds were sulfated in halophytic species, were positively correlated with salinity. We found that sulfated polysaccharides produced by Ruppia maritima Loisel disappeared when the plant was cultivated in the absence of salt. However, subjecting the glycophyte Oryza sativa Linnaeus to salt stress did not induce the biosynthesis of sulfated polysaccharides but increased the concentration of the carboxylated polysaccharides; this finding suggests that negatively charged cell wall polysaccharides might play a role in coping with salt stress. These data suggest that the presence of sulfated polysaccharides in plants is an adaptation to high salt environments, which may have been conserved during plant evolution from marine green algae. Our results address a practical biological concept; additionally, we suggest future strategies that may be beneficial when engineering salt-resistant crops.
Conflict of interest statement
Figures



References
-
- Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25:239–250. - PubMed
-
- FAO. Land and Plant Nutrition Management Service. 2008. http://www.fao.org/ag/agl/agll/spush.
-
- Yamaguchi T, Blumwald E. Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci. 2005;10:615–620. - PubMed
-
- Ashraf M, Akram NA. Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnol Adv. 2009;27:744–752. - PubMed
-
- Flowers TJ. Improving crop salt tolerance. J Exp Bot. 2004;55:307–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

