Stage-specific pathways of Leishmania infantum chagasi entry and phagosome maturation in macrophages
- PMID: 21552562
- PMCID: PMC3084250
- DOI: 10.1371/journal.pone.0019000
Stage-specific pathways of Leishmania infantum chagasi entry and phagosome maturation in macrophages
Abstract
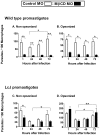
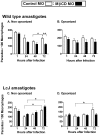
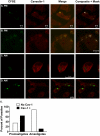
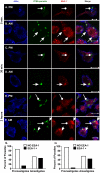
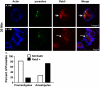
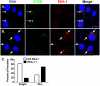
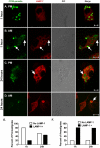
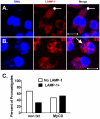
The life stages of Leishmania spp. include the infectious promastigote and the replicative intracellular amastigote. Each stage is phagocytosed by macrophages during the parasite life cycle. We previously showed that caveolae, a subset of cholesterol-rich membrane lipid rafts, facilitate uptake and intracellular survival of virulent promastigotes by macrophages, at least in part, by delaying parasitophorous vacuole (PV)-lysosome fusion. We hypothesized that amastigotes and promastigotes would differ in their route of macrophage entry and mechanism of PV maturation. Indeed, transient disruption of macrophage lipid rafts decreased the entry of promastigotes, but not amastigotes, into macrophages (P<0.001). Promastigote-containing PVs were positive for caveolin-1, and co-localized transiently with EEA-1 and Rab5 at 5 minutes. Amastigote-generated PVs lacked caveolin-1 but retained Rab5 and EEA-1 for at least 30 minutes or 2 hours, respectively. Coinciding with their conversion into amastigotes, the number of promastigote PVs positive for LAMP-1 increased from 20% at 1 hour, to 46% by 24 hours, (P<0.001, Chi square). In contrast, more than 80% of amastigote-initiated PVs were LAMP-1+ at both 1 and 24 hours. Furthermore, lipid raft disruption increased LAMP-1 recruitment to promastigote, but not to amastigote-containing compartments. Overall, our data showed that promastigotes enter macrophages through cholesterol-rich domains like caveolae to delay fusion with lysosomes. In contrast, amastigotes enter through a non-caveolae pathway, and their PVs rapidly fuse with late endosomes but prolong their association with early endosome markers. These results suggest a model in which promastigotes and amastigotes use different mechanisms to enter macrophages, modulate the kinetics of phagosome maturation, and facilitate their intracellular survival.
Conflict of interest statement
Figures
Similar articles
-
Biogenesis of Leishmania-harbouring parasitophorous vacuoles following phagocytosis of the metacyclic promastigote or amastigote stages of the parasites.J Cell Sci. 2002 Jun 1;115(Pt 11):2303-16. doi: 10.1242/jcs.115.11.2303. J Cell Sci. 2002. PMID: 12006615
-
Differences in human macrophage receptor usage, lysosomal fusion kinetics and survival between logarithmic and metacyclic Leishmania infantum chagasi promastigotes.Cell Microbiol. 2009 Dec;11(12):1827-41. doi: 10.1111/j.1462-5822.2009.01374.x. Epub 2009 Aug 20. Cell Microbiol. 2009. PMID: 19702651 Free PMC article.
-
Complement receptor 3 mediates ruffle-like, actin-rich aggregates during phagocytosis of Leishmania infantum metacyclics.Exp Parasitol. 2021 Jan;220:107968. doi: 10.1016/j.exppara.2020.107968. Epub 2020 Aug 8. Exp Parasitol. 2021. PMID: 32781093 Free PMC article.
-
Life in vacuoles--nutrient acquisition by Leishmania amastigotes.Int J Parasitol. 2001 Oct;31(12):1311-20. doi: 10.1016/s0020-7519(01)00259-4. Int J Parasitol. 2001. PMID: 11566299 Review.
-
Leishmania promastigotes: building a safe niche within macrophages.Front Cell Infect Microbiol. 2012 Sep 19;2:121. doi: 10.3389/fcimb.2012.00121. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 23050244 Free PMC article. Review.
Cited by
-
The macrophage microtubule network acts as a key cellular controller of the intracellular fate of Leishmania infantum.PLoS Negl Trop Dis. 2020 Jul 28;14(7):e0008396. doi: 10.1371/journal.pntd.0008396. eCollection 2020 Jul. PLoS Negl Trop Dis. 2020. PMID: 32722702 Free PMC article.
-
Leishmania donovani Internalizes into Host Cells via Caveolin-mediated Endocytosis.Sci Rep. 2019 Sep 2;9(1):12636. doi: 10.1038/s41598-019-49007-1. Sci Rep. 2019. PMID: 31477757 Free PMC article.
-
The Leishmania Parasitophorous Vacuole Membrane at the Parasite-Host Interface.Yale J Biol Med. 2019 Sep 20;92(3):511-521. eCollection 2019 Sep. Yale J Biol Med. 2019. PMID: 31543712 Free PMC article. Review.
-
Control of Phagocytosis by Microbial Pathogens.Front Immunol. 2017 Oct 24;8:1368. doi: 10.3389/fimmu.2017.01368. eCollection 2017. Front Immunol. 2017. PMID: 29114249 Free PMC article. Review.
-
Caveolae as Potential Hijackable Gates in Cell Communication.Front Cell Dev Biol. 2020 Oct 27;8:581732. doi: 10.3389/fcell.2020.581732. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195223 Free PMC article. Review.
References
-
- Basu MK, Ray M. Macrophage and Leishmania: an unacceptable coexistence. Crit Rev Microbiol. 2005;31:145–154. - PubMed
-
- McConville MJ, de Souza D, Saunders E, Likic VA, Naderer T. Living in a phagolysosome; metabolism of Leishmania amastigotes. Trends Parasitol. 2007;23:368–375. - PubMed
-
- de Freitas Balanco JM, Moreira ME, Bonomo A, Bozza PT, Amarante-Mendes G, et al. Apoptotic mimicry by an obligate intracellular parasite downregulates macrophage microbicidal activity. Curr Biol. 2001;11:1870–1873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

