Evaluation of lot-to-lot repeatability and effect of assay media choice in the recombinant Factor C assay
- PMID: 21552635
- PMCID: PMC4912006
- DOI: 10.1039/c1em10035a
Evaluation of lot-to-lot repeatability and effect of assay media choice in the recombinant Factor C assay
Abstract
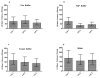
Measurement of environmental endotoxin exposures is complicated by variability encountered using current biological assay methods arising in part from lot-to-lot variability of the Limulus-amebocyte lysate (LAL) reagents. Therefore, we investigated the lot-to-lot repeatability of commercially available recombinant Factor C (rFC) kits as an alternative to LAL. Specifically, we compared endotoxin estimates obtained from rFC assay of twenty indoor dust samples, using four different extraction and assay media, to endotoxin estimates previously obtained by Limulus amebocyte lysate (LAL) assay and amounts of 3-hydroxy fatty acids (3-OHFA) in lipopolysaccharide (LPS) using gas-chromatography mass spectroscopy (GC-MS). We found that lot-to-lot variability of the rFC assay kits does not significantly alter endotoxin estimates in house dust samples when performed using three of the four assay media tested and that choice of assay media significantly altered endotoxin estimates obtained by rFC assay of house dust samples. Our findings demonstrate lot-to-lot reproducibility of rFC assay of environmental samples and suggest that use of rFC assay performed with Tris buffer or water as the extraction and assay medium for measurement of endotoxin in dust samples may be a suitable choice for developing a standardized methodology.
Figures


References
-
- Milton DK. American Conference of Governmental Industrial Hygienists, editor. In: Bioaerosol assessment and control. Macher J MD, Burge HA, Morey P, editors. Cincinnati, OH: 1999.
-
- Milton DK. In: Bioaerosols. Burge HA, editor. Lewis Publishers; Chelsea, MI: 1995. pp. 77–86.
-
- Wan GH, Li CS. Arch Environ Health. 1999;54:172–179. - PubMed
-
- Park JH, Gold DR, Spiegelman DL, Burge HA, Milton DK. Am J Respir Crit Care Med. 2001;163:322–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

