Parkinson's disease-linked LRRK2 is expressed in circulating and tissue immune cells and upregulated following recognition of microbial structures
- PMID: 21552986
- PMCID: PMC3376651
- DOI: 10.1007/s00702-011-0653-2
Parkinson's disease-linked LRRK2 is expressed in circulating and tissue immune cells and upregulated following recognition of microbial structures
Abstract
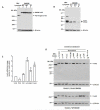
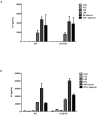
Sequence variants at or near the leucine-rich repeat kinase 2 (LRRK2) locus have been associated with susceptibility to three human conditions: Parkinson's disease (PD), Crohn's disease and leprosy. As all three disorders represent complex diseases with evidence of inflammation, we hypothesized a role for LRRK2 in immune cell functions. Here, we report that full-length Lrrk2 is a relatively common constituent of human peripheral blood mononuclear cells (PBMC) including affinity isolated, CD14(+) monocytes, CD19(+) B cells, and CD4(+) as well as CD8(+) T cells. Up to 26% of PBMC from healthy donors and up to 43% of CD14(+) monocytes were stained by anti-Lrrk2 antibodies using cell sorting. PBMC lysates contained full-length (>260 kDa) and higher molecular weight Lrrk2 species. The expression of LRRK2 in circulating leukocytes was confirmed by microscopy of human blood smears and in sections from normal midbrain and distal ileum. Lrrk2 reactivity was also detected in mesenteric lymph nodes and spleen (including in dendritic cells), but was absent in splenic mononuclear cells from lrrk2-null mice, as expected. In cultured bone marrow-derived macrophages from mice we made three observations: (i) a predominance of higher molecular weight lrrk2; (ii) the reduction of autophagy marker LC3-II in (R1441C)lrrk2-mutant cells (<31%); and (iii) a significant up-regulation of lrrk2 mRNA (>fourfold) and protein after exposure to several microbial structures including bacterial lipopolysaccharide and lentiviral particles. We conclude that Lrrk2 is a constituent of many cell types in the immune system. Following the recognition of microbial structures, stimulated macrophages respond with altered lrrk2 gene expression. In the same cells, lrrk2 appears to co-regulate autophagy. A pattern recognition receptor-type function for LRRK2 could explain its locus' association with Crohn's disease and leprosy risk. We speculate that the role of Lrrk2 in immune cells may also be relevant to the susceptibility of developing PD or its progression.
Figures



References
-
- Alcaïs A, Mira M, Casanova JL, Schurr E, Abel L. Genetic dissection of immunity in leprosy. Curr Opin Immunol. 2005;17:44–48. - PubMed
-
- Aleyasin H, Rousseaux MW, Marcogliese PC, Hewitt SJ, Irrcher I, Joselin AP, Parsanejad M, Kim RH, Rizzu P, Callaghan SM, Slack RS, Mak TW, Park DS. DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci USA. 2010;107:3186–3191. - PMC - PubMed
-
- Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, NIDDK IBD Genetics Consortium. Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Belgian-French IBD Consortium. Wellcome Trust Case Control Consortium. Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955–962. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

