Matrix proteins are inefficiently imported into Arabidopsis peroxisomes lacking the receptor-docking peroxin PEX14
- PMID: 21553312
- PMCID: PMC3529590
- DOI: 10.1007/s11103-011-9782-0
Matrix proteins are inefficiently imported into Arabidopsis peroxisomes lacking the receptor-docking peroxin PEX14
Abstract
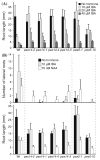
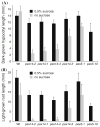
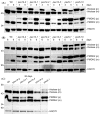
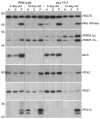
Mutations in peroxisome biogenesis proteins (peroxins) can lead to developmental deficiencies in various eukaryotes. PEX14 and PEX13 are peroxins involved in docking cargo-receptor complexes at the peroxisomal membrane, thus aiding in the transport of the cargo into the peroxisomal matrix. Genetic screens have revealed numerous Arabidopsis thaliana peroxins acting in peroxisomal matrix protein import; the viable alleles isolated through these screens are generally partial loss-of-function alleles, whereas null mutations that disrupt delivery of matrix proteins to peroxisomes can confer embryonic lethality. In this study, we used forward and reverse genetics in Arabidopsis to isolate four pex14 alleles. We found that all four alleles conferred reduced PEX14 mRNA levels and displayed physiological and molecular defects suggesting reduced but not abolished peroxisomal matrix protein import. The least severe pex14 allele, pex14-3, accumulated low levels of a C-terminally truncated PEX14 product that retained partial function. Surprisingly, even the severe pex14-2 allele, which lacked detectable PEX14 mRNA and PEX14 protein, was viable, fertile, and displayed residual peroxisome matrix protein import. As pex14 plants matured, import improved. Together, our data indicate that PEX14 facilitates, but is not essential for peroxisomal matrix protein import in plants.
Figures



Similar articles
-
Reducing PEX13 expression ameliorates physiological defects of late-acting peroxin mutants.Traffic. 2011 Jan;12(1):121-34. doi: 10.1111/j.1600-0854.2010.01136.x. Epub 2010 Nov 24. Traffic. 2011. PMID: 20969679 Free PMC article.
-
The E3 ubiquitin ligase SP1-like 1 plays a positive role in peroxisome biogenesis in Arabidopsis.Plant J. 2018 Jun;94(5):836-846. doi: 10.1111/tpj.13900. Epub 2018 Apr 24. Plant J. 2018. PMID: 29570879
-
Peroxisomal ubiquitin-protein ligases peroxin2 and peroxin10 have distinct but synergistic roles in matrix protein import and peroxin5 retrotranslocation in Arabidopsis.Plant Physiol. 2014 Nov;166(3):1329-44. doi: 10.1104/pp.114.247148. Epub 2014 Sep 11. Plant Physiol. 2014. PMID: 25214533 Free PMC article.
-
Peroxisome biogenesis.Rev Physiol Biochem Pharmacol. 2003;147:75-121. doi: 10.1007/s10254-003-0007-z. Epub 2003 Mar 25. Rev Physiol Biochem Pharmacol. 2003. PMID: 12687401 Review.
-
Peroxisome biogenesis, protein targeting mechanisms and PEX gene functions in plants.Biochim Biophys Acta. 2016 May;1863(5):850-62. doi: 10.1016/j.bbamcr.2015.09.027. Epub 2015 Sep 25. Biochim Biophys Acta. 2016. PMID: 26408938 Review.
Cited by
-
Pex13 and Pex14, the key components of the peroxisomal docking complex, are required for peroxisome formation, host infection and pathogenicity-related morphogenesis in Magnaporthe oryzae.Virulence. 2019 Dec;10(1):292-314. doi: 10.1080/21505594.2019.1598172. Virulence. 2019. PMID: 30905264 Free PMC article.
-
Peroxisome Function, Biogenesis, and Dynamics in Plants.Plant Physiol. 2018 Jan;176(1):162-177. doi: 10.1104/pp.17.01050. Epub 2017 Oct 11. Plant Physiol. 2018. PMID: 29021223 Free PMC article. Review.
-
E3 ubiquitin ligase SP1 regulates peroxisome biogenesis in Arabidopsis.Proc Natl Acad Sci U S A. 2016 Nov 15;113(46):E7307-E7316. doi: 10.1073/pnas.1613530113. Epub 2016 Oct 31. Proc Natl Acad Sci U S A. 2016. PMID: 27799549 Free PMC article.
-
The Roles of β-Oxidation and Cofactor Homeostasis in Peroxisome Distribution and Function in Arabidopsis thaliana.Genetics. 2016 Nov;204(3):1089-1115. doi: 10.1534/genetics.116.193169. Epub 2016 Sep 7. Genetics. 2016. PMID: 27605050 Free PMC article.
-
A PEX5 missense allele preferentially disrupts PTS1 cargo import into Arabidopsis peroxisomes.Plant Direct. 2019 Mar;3(3):e00128. doi: 10.1002/pld3.128. Epub 2019 Mar 20. Plant Direct. 2019. PMID: 31236542 Free PMC article.
References
-
- Albertini M, Rehling P, Erdmann R, Girzalsky W, Kiel JA, Veenhuis M, Kunau WH. Pex14p, a peroxisomal membrane protein binding both receptors of the two PTS-dependent import pathways. Cell. 1997;89:83–92. - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, Jeske A, Koesema E, Meyers CC, Parker H, Prednis L, Ansari Y, Choy N, Deen H, Geralt M, Hazari N, Hom E, Karnes M, Mulholland C, Ndubaku R, Schmidt I, Guzman P, Aguilar-Henonin L, Schmid M, Weigel D, Carter DE, Marchand T, Risseeuw E, Brogden D, Zeko A, Crosby WL, Berry CC, Ecker JR. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. - PubMed
-
- Azevedo JE, Schliebs W. Pex14p, more than just a docking protein. Biochim Biophys Acta. 2006;1763:1574–1584. - PubMed
-
- Baker A, Graham IA, Holdsworth M, Smith SM, Theodoulou FL. Chewing the fat: β-oxidation in signalling and development. Trends Plant Sci. 2006;11:124–132. - PubMed
-
- Bell CJ, Ecker JR. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

