On the mechanism of the palladium-catalyzed tert-butylhydroperoxide-mediated Wacker-type oxidation of alkenes using quinoline-2-oxazoline ligands
- PMID: 21553838
- PMCID: PMC3113657
- DOI: 10.1021/ja2017043
On the mechanism of the palladium-catalyzed tert-butylhydroperoxide-mediated Wacker-type oxidation of alkenes using quinoline-2-oxazoline ligands
Abstract
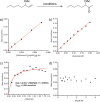
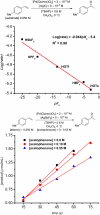
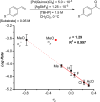
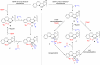
The mechanism of the tert-butylhydroperoxide-mediated, Pd(Quinox)-catalyzed Wacker-type oxidation was investigated to evaluate the hypothesis that a selective catalyst-controlled oxidation could be achieved by rendering the palladium coordinatively saturated using a bidentate amine ligand. The unique role of the Quinox ligand framework was probed via systematic ligand modifications. The modified ligands were evaluated through quantitative Hammett analysis, which supports a "push-pull" relationship between the electronically asymmetric quinoline and oxazoline ligand modules.
© 2011 American Chemical Society
Figures

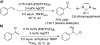
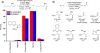
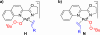
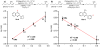



References
-
- Tsuji J, Nagashima H, Nemoto H. Org. Synth. 1984;62:9–13.
-
- Tsuji J. Synthesis. 1984:369–384.
-
- Takacs JM, Jiang X.-t. Curr. Org. Chem. 2003;7:369–396.
-
- Cornell CN, Sigman MS. Inorg. Chem. (Washington, DC, U. S.) 2007;46:1903–1909. - PubMed
-
- Kang S-K, Jung K-Y, Chung J-U, Namkoong E-Y, Kim T-H. J. Org. Chem. 1995;60:4678–4679.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

