Conformational and aggregation properties of a PEGylated alanine-rich polypeptide
- PMID: 21553871
- PMCID: PMC3114202
- DOI: 10.1021/bm200272w
Conformational and aggregation properties of a PEGylated alanine-rich polypeptide
Abstract
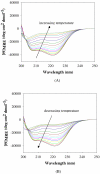
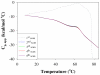
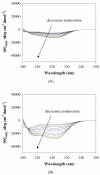
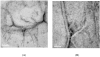
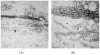
The conformational and aggregation behavior of PEG conjugates of an alanine-rich polypeptide (PEG-c17H6) were investigated and compared to that of the polypeptide equipped with a deca-histidine tag (17H6). These polypeptides serve as simple and stimuli-responsive models for the aggregation behavior of helix-rich proteins, as our previous studies have shown that the helical 17H6 self-associates at acidic pH and converts to β-sheet structures at elevated temperature under acidic conditions. In the work here, we show that PEG-c17H6 also adopts a helical structure at ambient/subambient temperatures, at both neutral and acidic pH. The thermal denaturation behavior of 17H6 and PEG-c17H6 is similar at neutral pH, where the alanine-rich domain has no self-association tendency. At acidic pH and elevated temperature, however, PEGylation slows β-sheet formation of c17H6, and reduces the apparent cooperativity of thermally induced unfolding. Transmission electron microscopy of PEG-c17H6 conjugates incubated at elevated temperatures showed fibrils with widths of ∼20-30 nm, wider than those observed for fibrils of 17H6. These results suggest that PEGylation reduces β-sheet aggregation in these polypeptides by interfering, only after unfolding of the native helical structure, with interprotein conformational changes needed to form β-sheet aggregates.
Figures




References
-
- Pavlou AK, Reichert JM. Nat. Biotechnol. 2004;22:1513–1519. - PubMed
-
- Roque ACA, Lowe CR, Taipa MA. Biotechnol. Prog. 2004;20:639–654. - PubMed
-
- Leader B, Baca QJ, Golan DE. Nat. Rev. Drug Discovery. 2008;7:21–39. - PubMed
-
- Huang CJ, Lowe AJ, Batt CA. Appl. Microbiol. Biotechnol. 2010;87:401–410. - PubMed
-
- Andya JD, Maa YF, Costantino HR, Nguyen PA, Dasovich N, Sweeney TD, Hsu CC, Shire SJ. Pharm. Res. 1999;16:350–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

