Nickel-catalyzed Kumada cross-coupling reactions of tertiary alkylmagnesium halides and aryl bromides/triflates
- PMID: 21553878
- PMCID: PMC8036247
- DOI: 10.1021/ja202769t
Nickel-catalyzed Kumada cross-coupling reactions of tertiary alkylmagnesium halides and aryl bromides/triflates
Abstract
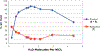
We report a Ni-catalyzed process for the cross-coupling of tertiary alkyl nucleophiles and aryl bromides. This process is extremely general for a wide range of electrophiles and generally occurs with a ratio of retention to isomerization >30:1. The same procedure also accommodates the use of aryl triflates, vinyl chlorides, and vinyl bromides as the electrophilic component.
Figures
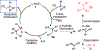

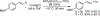
References
-
- de Meijere A, Diederich F, Eds. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: New York, 2004.
- Rudolph A; Lautens M Angew. Chem., Int. Ed 2009, 48, 2656 and references cited therein. - PubMed
-
- Chemler SR; Trauner D; Danishefsky SJ Angew. Chem., Int. Ed 2001, 40, 4544. - PubMed
- Netherton MR; Fu GC In Topics in Organometallic Chemistry: Palladium in Organic Synthesis; Tsuji J, Ed.; Springer: New York, 2005; pp 85–108.
-
-
For recent examples of secondary nucleophiles in Pd catalysis:
- Campos KR; Klapars A; Walsman JH; Dormer PG; Chen CJ Am. Chem. Soc 2006, 128, 3538. - PubMed
- Luo X; Zhang H; Duan H; Liu Q; Zhu L; Zhang T; Lei A Org. Lett 2007, 9, 4571. - PubMed
- Dreher SD; Dormer PG; Sandrock DL; Molander GA J. Am. Chem. Soc 2008, 130, 9257. - PMC - PubMed
- Han C; Buchwald SL J. Am. Chem. Soc 2009, 131, 7532. - PMC - PubMed
- Thaler T; Haag B; Gavryushin A; Schober K; Hartman E; Gschwing RM; Zipse H; Mayer P; Knochel P Nature Chem. 2010, 2, 125. - PubMed
- Nakao Y; Takeda M; Matsumoto T; Hiyama T Angew. Chem., Int. Ed 2010, 45, 4447. - PubMed
- Sandrock DL; Jean-Gerard L; Chen C-Y; Dreher SD; Molander GA J. Am. Chem. Soc 2010, 132, 17108. - PMC - PubMed
-
For recent examples of secondary nucleophiles in Ni catalysis:
- Melzig L; Gavryushin A; Knochel P Org. Lett 2007, 9, 5529. - PubMed
- Smith SW; Fu GC Angew. Chem., Int. Ed 2008, 47, 9334. - PMC - PubMed
- Phapale VB; Guisan-Ceinos M; Bunuel E; Cardenas DJ Chem.—Eur. J 2009, 15, 12681. - PubMed
- Joshi-Pangu A; Ganesh M; Biscoe MR Org. Lett 2011, 13, 1218. - PMC - PubMed
-
-
-
While a Ni(0)–Ni(II) catalytic cycle cannot be conclusively ruled out, we favor a Ni(I)–Ni(III) catalytic cycle for such Ni-catalyzed reactions. See:
- Phapale VB; Guisan-Ceinos M; Bunuel E; Cardenas DJ Chem.—Eur. J 2009, 15, 12681. - PubMed
- Anderson TJ; Jones GD; Vicic DA J. Am. Chem. Soc 2004, 126, 8100. - PubMed
- Jones GD; Martin JL; McFarland C; Allen OR; Hall RE; Haley AD; Brandon RJ; Konovalova T; Desrochers PJ; Pulay P; Vicic DA J. Am. Chem. Soc 2006, 128, 13175. - PubMed
-
-
- Luo X; Zhang H; Duan H; Liu Q; Zhu L; Zhang T; Lei A Org. Lett 2007, 9, 4571. - PubMed
- Breitenfeld J; Vechorkin O; Corminboeuf C; Scopelliti R; Hu X Organometallics 2010, 29, 3686.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

