Relaxin promotes growth and maturation of mouse neonatal cardiomyocytes in vitro: clues for cardiac regeneration
- PMID: 21554533
- PMCID: PMC3822927
- DOI: 10.1111/j.1582-4934.2011.01328.x
Relaxin promotes growth and maturation of mouse neonatal cardiomyocytes in vitro: clues for cardiac regeneration
Abstract
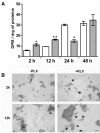
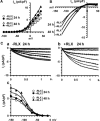
The demonstration that the adult heart contains myocardial progenitor cells which can be recruited in an attempt to replace the injured myocardium has sparkled interest towards novel molecules capable of improving the differentiation of these cells. In this context, the peptide hormone relaxin (RLX), recently validated as a cardiovascular hormone, is a promising candidate. This study was designed to test the hypothesis that RLX may promote the growth and maturation of mouse neonatal immature cardiomyocytes in primary culture. The cultures were studied at 2, 12, 24 and 48 hrs after the addition of human recombinant H2 RLX (100 ng/ml), the main circulating form of the hormone, or plain medium by combining molecular biology, morphology and electrophysiology. RLX modulated cell proliferation, promoting it at 2 and 12 hrs and inhibiting it at 24 hrs; RLX also induced the expression of both cardiac-specific transcription factors (GATA-4 and Nkx2-5) and cardiac-specific structural genes (connexin 43, troponin T and HCN4 ion channel) at both the mRNA and protein level. Consistently, RLX induced the appearance of ultrastructural and electrophysiological signs of functionally competent, mature cardiomyocytes. In conclusion, this study provides novel circumstantial evidence that RLX specifically acts on immature cardiomyocytes by promoting their proliferation and maturation. This notion suggests that RLX, for which the heart is both a source and target organ, may be an endogenous regulator of cardiac morphogenesis during pre-natal life and could participate in heart regeneration and repair, both as endogenous myocardium-derived factor and exogenous cardiotropic drug, during adult life.
© 2012 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures




References
-
- Hellermann JP, Jacobsen SJ, Gersh BJ, et al. Heart failure after myocardial infarction: a review. Am J Med. 2002;113:324–30. - PubMed
-
- Formigli L, Zecchi-Orlandini S, Meacci E, et al. Skeletal myoblasts for heart regeneration and repair: state of the art and perspectives on the mechanisms for functional cardiac benefits. Curr Pharm Des. 2010;16:915–28. - PubMed
-
- Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. - PubMed
-
- Urbanek K, Rota M, Cascapera S, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

