A double-blind randomized controlled trial to study the efficacy of topiramate in a civilian sample of PTSD
- PMID: 21554564
- PMCID: PMC6493911
- DOI: 10.1111/j.1755-5949.2010.00188.x
A double-blind randomized controlled trial to study the efficacy of topiramate in a civilian sample of PTSD
Abstract
Objective: To evaluate the efficacy and tolerability of topiramate in patients with posttraumatic stress disorder (PTSD).
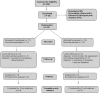
Method: We conducted a 12-week double-blind, randomized, placebo-controlled study comparing topiramate to placebo. Men and women aged 18-62 years with diagnosis of PTSD according to DSM-IV were recruited from the outpatient clinic of the violence program of Federal University of São Paulo Hospital (Prove-UNIFESP), São Paulo City, between April 2006 and December 2009. Subjects were assessed for the Clinician-Administered Posttraumatic Stress Scale (CAPS), Clinical Global Impression, and Beck Depression Inventory (BDI). After 1-week period of washout, 35 patients were randomized to either group. The primary outcome measure was the CAPS total score changes from baseline to the endpoint.
Results: 82.35% of patients in the topiramate group exhibited improvements in PTSD symptoms. The efficacy analysis demonstrated that patients in the topiramate group exhibited significant improvements in reexperiencing symptoms: flashbacks, intrusive memories, and nightmares of the trauma (CAPS-B; P= 0.04) and in avoidance/numbing symptoms associated with the trauma, social isolation, and emotional numbing (CAPS-C; P= 0.0001). Furthermore, the experimental group demonstrated a significant difference in decrease in CAPS total score (topiramate -57.78; placebo -32.41; P= 0.0076). Mean topiramate dose was 102.94 mg/d. Topiramate was generally well tolerated.
Conclusion: Topiramate was effective in improving reexperiencing and avoidance/numbing symptom clusters in patients with PTSD. This study supports the use of anticonvulsants for the improvement of symptoms of PTSD.
© 2010 Blackwell Publishing Ltd.
Conflict of interest statement
The authors have no conflict of interest.
Figures
References
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders, 3rd ed. Washington DC : APA, 1980.
-
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995;52:1048–1060. - PubMed
-
- Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder. Cochrane Database Syst Rev 2006;25:CD 002795.
-
- American Psychiatric Association . Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Washington , DC : APA, 2004. - PubMed
-
- Post RM, Weiss SR, Smith M, Li H, McCann U. Kindling versus quenching. Implications for the evolution and treatment of posttraumatic stress disorder. Ann N Y Acad Sci 1997;821:285–295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous